Int J Aging. 2023;1:e12.
doi: 10.34172/ija.2023.e12
Review Article
The Role of Gut Microbiota in Aging-Associated Diseases
Ahmad Mobed 1, * 
Author information:
1Department of Microbiology, School of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Objectives:
To review gut microbiota in aging-associated diseases.
Design:
A review study.
Participants:
People over 60 years of age with microbiota dysbiosis.
Outcome measures:
The occurrence of aging-associated diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), osteoarthritis (OA), prostate cancer (PC), and colorectal cancer (CRC).
Results:
The microbiome plays an essential role in the maturation, function, and regulation of human life from birth to old age. Human life, in turn, has co-evolved interactions with the trillions of beneficial microbes that inhabit our bodies while developing efficient responses to combat invading pathogens. Along with this, both human life and the gut microbiota (GM) undergo major modifications in conformation and function that resulted in increased vulnerability to infections and other age-related diseases such as Parkinson, Alzheimer, and OA.
Conclusions:
The GM is involved in a variety of physiological and pathological processes. Its role in age-related diseases is well recognized and has been identified as a promising therapeutic target. Moreover, the microbiota of the elderly population exhibits unique microbial signatures that link the natural aging process to changes in the composition of the GM.
Keywords: Aging, Microbiome, Gut microbiota, Dysbiosis, Parkinson’s disease, Alzheimer’s disease
Introduction
According to estimates made, one-sixth of the world’s population will be over 60 years old by 2030.1 In other words, the proportion of the population over 60 will increase from 1 billion in 2020 to 1.4 billion.1 Subsequently, the world’s population over 60 years will reach more than double (2.1 billion) by 2050. Furthermore, between 2020-2050, the population over 80 years old is expected to triple and reach more than 426 million people.2 Aging is associated with modifications in physiological, dynamic, biological, environmental, behavioral, psychological, and social progressions.3,4 Some age-related changes are benign, while others result in impaired activity and sensory function of daily life, increased susceptibility to and incidence of disease, and disability. In fact, aging is a major risk factor for many chronic diseases.5 The human body is colonized by trillions of microbial cells during all parts of life.6 The collection of all microorganisms, their environmental conditions, genes, and the inside and outside the human body, called the human microbiome, make up the human ecosystem. Everyone has an exclusive microbiome that becomes more and more unique with age.5,7 This may reflect the highest lifetime interactions, including demographic and environmental influences. These diverse microbes typically coexist pleasantly with their hosts and, in some cases, help maintain their health and immune function which may prevent disease progression.8 The gut microbiota (GM) plays an important role in maintaining host local and systemic physiology.8,9 In other words, the various beneficial functions of GM include nutrient metabolism, intestinal homeostasis, maintenance of immune system homeostasis, immune regulation of the host digestive system, and intestinal mucosal development and metabolic activity.10,11 Different studies have shown that the population of gut microorganisms changes during the lifetime. For example, findings revealed that Clostridium clostridioforme, Finegoldia magna, and Bifidobacterium dentium were amplified abundantly in the elderly.12 The factors that lead to changes in microbiota composition and function with host aging are largely unknown and involve direct or indirect microbial selection by host-microbe interactions and microbial evolution.13 The main focus of the current study was to introduce diseases related to GM in old age. In addition, this study described microbial types and key biomarkers of disease. In other words, readers of this overview would gain valuable information about diseases that are common in older adults such as Parkinson’s disease (PD), Alzheimer’s disease (AD), arthritis, and prostate cancer (PC) as well as their relationship to the gut microbiome.
Methods
PubMed databank was searched to recognize publications from peer-reviewed journals. We used Medical Subject Heading terms, including, ‘aging’, ‘microbiome’, ‘microbiota,’ ‘dysbiosis’, and ‘diseases’ in combination with further free terms such as ‘Parkinson’s disease’ ‘Alzheimer’s disease’, ‘osteoarthritis’, ‘colorectal cancer’, ‘prostate cancer’, and ‘elderly’. Furthermore, medRxiv (https://www.medrxiv.org/) was investigated for pre-print articles with ‘aging AND microbiota’ keywords. The search was conducted on March 11, 2023, and no search filters on publication type, language, or other field’s expected time periods were employed. Reference lists of all relevant publications were then manually selected to identify advanced qualified studies.
Results
Aging-Associated Diseases
Aging is an irreversible and gradual pathophysiological process.14 A decline in cell and tissue function greatly increases the risk of several age-related diseases, including cardiovascular, neurodegenerative, metabolic, immune, and musculoskeletal diseases.15,16 The progress in present medicine has encouraged human health and significantly improved life expectancy, but with population aging, various chronic diseases have increasingly become the main cause of infirmity and mortality among the elderly.16,17 Considering the multifactorial nature of aging and its direct relationship with environmental and genetic factors (e.g., the accumulation of DNA damage, telomere shortening, and metabolic changes), modifying the molecular mechanisms that cause aging is a difficult task.18,19 On the other hand, epigenetic regulators with genetic and environmental influences at the cellular, molecular, and systemic levels respond to stress through complex molecular mechanisms and the overproduction of reactive oxygen species and have synergistic effects on aging.20,21 Collectively, these mechanisms prevent cells from altering metabolic and gene expression patterns, induce high reactive oxygen species production, and maintain the senescent phenotype of cells.22 The results of various studies demonstrated that the above modifications and molecular mechanisms are directly related to the occurrence of aging-related diseases.22 Meanwhile, the microbiome is one of the important factors that directly or indirectly plays a critical role in the occurrence of molecular changes and subsequently the occurrence of various diseases related to old age.23 The role and relationship of microbiota with various age-related diseases have been fully determined although the involved molecular mechanisms are somewhat unknown.24 Alzheimer’s, Parkinson’s, osteoarthritis (OA), and some metabolic diseases such as diabetes are among the important diseases related to the microbiome in old age.24 In the following, more details about the relationship between the microbiome and these diseases will be discussed.
Microbiome Evolution and Related Disorders in Life Time
The composition of the adult microbiome is individually specific, showing a persistent trend towards a core microbiome with aging.25 The exact composition of the microbiome in older adults (aged over 65) can exhibit extreme short-term fluctuations. The core microbiome appears to be resistant to environmental changes.26 Despite some primary evidence to the contrary, it is clear that the host contributes to the selection of microbiome composition.27,28 Microbiota development is widely believed to begin at birth, but this episode is challenged by the limited number of studies that have identified microbes in uterine tissues such as the placenta.29 Colonization and life events such as antibiotic treatment, disease, and dietary changes cause unregulated changes in the microbiota.30 High levels of lactobacilli during the first few days reflect a high lactobacilli load of the vaginal flora.31 Accordingly, the finding revealed that Bifidobacterium longum subspecies longum subspecies infantis are dominant in infants.32 Accordingly, exposing the infant to antibiotics via the umbilical cord affects the infant’s gut microbiome and not only reduces beneficial commensal organisms such as Bifidobacteria but also increases potential pathogenic bacteria such as Escherichia coli and Enterococci.33 Neonatal disorders mean the disruption of the normal state of the body and organs and the irregular function of a newborn. Obstetricians play a key role to reduce the number of neonatal disorders.34 Respiratory dysfunction, birth trauma, neonatal infection, congenital malformations, and hemolytic disorders of the newborn are some cases of frequently encountered neonatal disorders. The intestinal microbiota changes during human life are illustrated in Figure 1.35
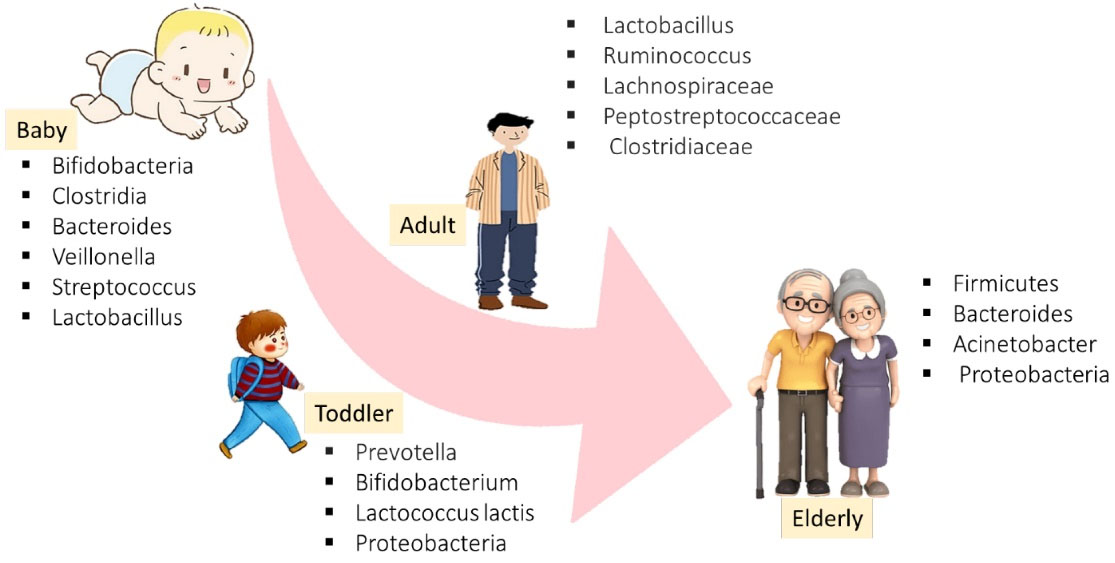
Figure 1.
The Human Microbiome and Its Onset and Development in Lifetime. Source. Satheesh et al36
.
The Human Microbiome and Its Onset and Development in Lifetime. Source. Satheesh et al36
Findings show that most changes occur with age, and this microbiota change is associated with the emergence of age-related diseases. It has become one of the research goals of researchers in recent years. In the following, the role of GM in aging-associated diseases is discussed.
Discussion
The Role of Gut Microbiota in Aging-Associated Diseases
Parkinson’s Disease
PD is characterized by the loss of dopaminergic neurons and intracellular inclusions composed primarily of α-synuclein (α-syn), but the underlying mechanisms are still unclear.37 During the last decade, several studies have focused on the relationship between the gut and PD pathology. Alterations in gut microbiome composition have been defined in many neurodegenerative disorders, of which PD has been considered most extensively.38 Several studies supported the idea that high consumption of milk derivatives, in general, is associated with an increased risk of PD.39-41 Several studies have been conducted that characterize changes in the gut microbiome. The findings revealed that abundance of the Bifidobacteriaceae, Pasteurellaceae, Lachnospiraceae Lactobacillaceae, Christensenellaceae, and Verrucomicrobiaceae families significantly alter in PD.42 Additionally, it has been confirmed that GM regulates neuroinflammation, synucleinopathy, and motor impairments in a rodent PD model.43 Microorganisms differ in their cellular architecture and tendency to initiate pattern recognition receptors in signaling pathways, leading to inflammation.44 It is suggested that improved concentrations of E. coli and the proteobacteria Ralstonia decrease plasma lipopolysaccharide-binding protein leading to higher endotoxin exposure and promoting intestinal inflammation.45 This inflammation is associated with the improved expression of the pro-inflammatory cytokines interferon-γ, interleukin-6, tumor necrosis factor-alpha, interleukin-1β, and amplified activation of intestinal glial cells consistent with colonic biopsies from PD patients.46 Scientists reported that short-chain fatty acids (SCFAs) are important metabolites of GM and that PD patients have lower fecal SCFA concentrations compared to healthy controls.45 Several studies have displayed a decrease in the frequency of Lachnospiraceae, known for their abundant production of SCFAs, in PD patients.47,48 In addition, SCFAs have been suggested to be a key factor in inducing microglial activation and accelerating α-synuclein damage in mouse models, thereby ameliorating PD pathophysiology.47,48 An important aspect of the interaction between the microbiome and host is the barrier function of the intestinal epithelium.49 Barrier disruption can generate a positive response loop relating intestinal reactive oxygen/nitrogen species, inflammation, in the intestinal lumen, and changes in microbial composition.50 The destabilization of the protective gastrointestinal barrier due to the translocation of bacteria or bacterial products such as lipopolysaccharides has a critical effect on the ‘microbiota–gut–brain axis’.50 This leads to intestinal inflammation and oxidative stress that induces enlarged α-syn aggregation and mucosal permeability in the enteric nervous system.51,52 Improved intestinal permeability, or intestinal leak, has been displayed in PD patients compared to mouse models and healthy controls of PD that correlate with tissue oxidative stress and increased intestinal α-syn deposition.50,53
Alzheimer’s Disease
AD is a progressive neurodegenerative disease characterized by the inability to perform daily activities, memory loss, dramatic personality and behavioral changes, and the late stages of the disease.54 An association has been verified between cerebral amyloidosis, inflammatory gut bacterial taxa, and peripheral inflammatory markers in people with cognitive impairment in old age.55 The results of this study showed that increased blood levels of pro-inflammatory cytokines such as interleukin-1β, interleukin-6, chemokine (C-X-C motif) ligand 2, and NLRP3 are associated with decreased levels of E. coli in dementia and amyloidosis patients.56 A positive relationship was correspondingly shown observed between the number of pro-inflammatory bacteria belonging to the taxon Escherichia/Shigella in fecal samples, and pro-inflammatory cytokines.56 A negative correlation was found between bacterium belonging to the taxonomic group of E. coli.56 A microbiological research established a reduction in several microorganisms as well as the Actinobacteria phyla, in particular, bacteria of the genus Bifidobacterium, and Firmicutes. Additionally, a proliferation of bacteria belonging to the Bacteroidetes and Proteobacteria phyla was provided successfully in the intestinal microbiome of AD patients.57 Moreover, a study revealed substantial microbiome differences in AD patients’ bowels of taxonomic groups such as Ruminococcus, Lachnospiraceae, Bacteroides, Actinobacteria, and Selenomonadales.58 However, qualitative changes in the GM of AD patients were somewhat different compared to healthy controls. Furthermore, the number of Bacteroidetes strains decreased, whereas that of Firmicutes strains did not change compared to healthy subjects. These variances could be associated with many factors, including comorbidities, culture, lifestyle, and dietary favorites.59 GM metabolites such as SCFAs, trimethylamine-n-oxide, and lipopolysaccharide are recommended to mediate systemic intracerebral amyloidosis and inflammation via endothelial dysfunction. Developing data suggests that the fungal microbiota could also influence AD pathology.60 The involvement of the GM in the development and progression of AD has been demonstrated, but despite early evidence of the involvement of inflammatory pathways, its precise role has not been described. Given the reported changes in GM of patients with AD, phylum replacement may have therapeutic implications.61
Osteoarthritis
OA is an important degenerative joint disease, affecting an estimated 18% of women and 10% of men worldwide, representing 60 million people across the world.62,63 These statistics are estimated to increase in the next years owing to the increasing occurrence of aging and obese populations, both of which are critical risk factors for OA.62,63 Findings indicated that the gut is a stimulating and innovative target for OA therapy. Nutritional variation or supplementation with prebiotics, fiber, or probiotics could exert a positive impact on the gut joint axis.64 Alterations in the microbiome are strongly associated with individual OA risk factors related to both the OA disease process and microbial DNA patterns in the gut microbiome and joints. Microbiome-targeted interventions may prevent or reduce the progression of OA.65 Forthcoming works should explore the basis of these microbiome-associated mechanisms and describe the beneficial potential of microbiome enhancement.66 The original study showed an association between the plasma microbiome and serum lipopolysaccharides in obese OA patients, revealing altered intestinal permeability.67 Lactobacillus species (LA) are widely used probiotics with well-known anti-inflammatory and antibacterial effects. LA has also been presented to relieve pain and inhibit cartilage destruction in a chemically-induced OA animal model.68 Intestinal barrier dysfunction has similarly been described in OA. Conversely, it is not clear whether LA species can modify intestinal inflammation and restore the GM throughout OA treatment.68 Moreover, the finding demonstrated that the microbiome threatens joint tissue integrity during the OA disease progress, which was accompanied by important modifications in the OA gut microbiome.
Prostate Cancer
Emergent data confirming that the microbiome is involved in the progress and treatment of PC through two molecular pathways69: (A) direct effects of microorganisms or microbial metabolites on the prostate or urine and (B) indirect effects of microorganisms or microbial metabolites on the gastrointestinal tract.69 In addition, the GM may act as a source of testosterone that influences PC progression.70 Men with castration-resistant PC have enlarged amounts of GM with androgenic function.70 Furthermore, males with high-risk PC exhibited a specific gut microbial profile, and GM outlining could be an influential tool for screening males with high-risk PC.71 However, lifestyle changes can improve the gut flora, and altering the GM through prebiotic or probiotic interventions can prevent or delay the development of PC.72 Streptococcal andBacteroidesspecies were discovered as the most copious bacteria in patients with PC. Moreover, pathways related to arginine and folate metabolism were changed in patients with PC. Correspondingly, findings indicated the enhancement of Bacteroides in PC and Eubacterium andFaecalibacteriumin non-cancer controls.73 A microbiological study demonstrated that Rikenellaceae, Lachnospira, andAlistipes were considerably higher in those at a high risk of PC.74 In addition, 16S rRNA sequencing as a genetic-based technique indicated that Streptococcus and Bacteroides species are higher in males with PC.75 The regulatory mechanism of PC by the GM was mysterious until Japanese scientists newly found that antibiotic administration suppresses high-fat diet-induced PC growth in a Pten-knockout PC mouse model.76
Colorectal Cancer
Colorectal cancer (CRC) is the third usual cancer type and the fourth most common cause of cancer-related deaths.77 Most cases of CRC are identified in Western countries, and their incidence is growing year by year. The odds of developing CRC are approximately 4%-5%, and the risk of developing CRC is related to individual characteristics and habits such as age, chronic medical history, and lifestyle.78,79 In addition to dietary effects, some degrees of plasticity in the human gut ecosystem can be detected in response to less clear environmental stressors such as climate and geography as well as degrees of exposure to environmental bacteria.77,80 The latter is most important in upbringing and maintenance.77,80 Aging can directly affect the structure of the GM through age-related physiological processes, including local and systemic inflammation, and it can indirectly affect people, leading to dietary and lifestyle changes.79 More details of GM in aging-associated diseases are summarized in Table 1.
Table 1.
Gut Microbiota in Aging-associated Diseases
|
Disease
|
Microbiome
|
Key Biomarkers
|
Ref
|
| PD |
Akkermansia muciniphila, Prevotellaceae, Bifidobacteriaceae, Pasteurellaceae, Lachnospiraceae, Christensenellaceae, Lactobacillaceae, and Verrucomicrobiaceae |
α-Synuclein |
42,81
|
| PD |
GM |
Neuroinflammation, synucleinopathy |
43
|
| PD |
E. coli and the proteobacteria Ralstonia |
α-Synuclein, cytokines |
46
|
| PD |
Lachnospiraceae
|
SCFAs |
48
|
| PD |
GM |
α-Synuclein |
52
|
| AD |
Escherichia/Shigella
|
Amyloids, IL-1β, IL-6, CXCL2, and NLRP3 |
56
|
| AD |
Bifidobacterium, Firmicutes, Proteobacteria, Actinobacteria phyla and Bacteroidetes |
Amyloids |
57
|
| AD |
Ruminococcus, Lachnospiraceae, Bacteroides, Actinobacteria, and Selenomonadales |
Amyloids, metabolites |
58
|
| AD |
Bacteroidetes, Firmicutes
|
Amyloids, Metabolites |
59
|
| AD |
GM, fungal microbiota |
SCFAs, trimethylamine-n-oxide, and LPS |
60
|
| AD |
Bacteroidetes, Firmicutes
|
Amyloids, and metabolites |
61
|
| OA |
Microbiome |
Prebiotics, fiber, probiotics |
64
|
| OA |
Microbiome |
Related metabolites |
66
|
| OA |
L. acidophilus
|
Related metabolites |
68
|
| PC |
Microbiome, GM |
Related metabolites |
69
|
| PC |
Faecalibacteriumand Eubacterium |
Arginine and folate |
73
|
| PC |
Rikenellaceae, Lachnospira, andAlistipes |
SCFAs |
74
|
| PC |
Streptococcus and Bacteroides |
SCFAs |
75
|
| CRC |
Clostridium leptumand Clostridium coccoidessubgroups |
Related metabolites |
80
|
| CRC |
Bacteroides/ Prevotella
|
Related metabolites |
79
|
Note. PD: Parkinson’s disease; GM: Gut microbiota; E. coli: Escherichia coli; IL-1β: Interleukin-1β; IL-6: Interleukin-6; SCFAs: Short-chain fatty acids; AD: Alzheimer’s disease; OA: Osteoarthritis; L. acidophilus; Lactobacillus acidophilus; PC: Prostate cancer; LPS: Lipopolysaccharides; CXCL2: Chemokine (C-X-C motif) ligand 2; CRC: Colorectal cancer.
Conclusions
A growing body of experimental in vitro and in vivo animal studies and epidemiological evidence strongly recommend that gut microbiome influences the progression of diseases in the elderly such as PD and AD. Studies of the gut microbiome in aging-associated diseases are highly complex, indicating that numerous important confounding factors need to be cautiously considered in future studies, including studies on geographic/population and methodology differences. Although the association between aging diseases and microbiome appears modest, we were able to identify several consistent microbiota features with important clinical correlates. Overall, the results of this review suggest that GM composition changes in patients with aging, and changes in GM may influence disease progression. However, these results are fully correlated, and to assess whether GM alterations directly influence the pathogenesis of age-related diseases, results of therapeutic clinical trials on microbiome-mediated human aging are required. The GM is involved in a variety of physiological and pathological processes. Its role in age-related diseases is well recognized and has been identified as a promising therapeutic target. Moreover, the microbiota of the elderly population exhibits unique microbial signatures that link the natural aging process to changes in the composition of the GM.
Acknowledgments
This study was supported by the Department of Microbiology, School of Medicine, the Tabriz University of Medical Sciences, Tabriz, Iran, Aging Research Institute, and the Faculty of Medicine at the Tabriz University of Medical Sciences, Iran.
Funding
Not available.
Data availability statement
Not available.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interests or personal relationships that could apparently influence the work reported in this study.
References
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019; 157:107843. doi: 10.1016/j.diabres.2019.107843 [Crossref] [ Google Scholar]
-
https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z. Measuring biological aging in humans: a quest. Aging Cell 2020; 19(2):e13080. doi: 10.1111/acel.13080 [Crossref] [ Google Scholar]
- Scott AJ, Ellison M, Sinclair DA. The economic value of targeting aging. Nat Aging 2021; 1(7):616-23. doi: 10.1038/s43587-021-00080-0 [Crossref] [ Google Scholar]
- Townsend EC, Kalan LR. The dynamic balance of the skin microbiome across the lifespan. Biochem Soc Trans 2023; 51(1):71-86. doi: 10.1042/bst20220216 [Crossref] [ Google Scholar]
- Vemuri R, Herath MP. Beyond the gut, emerging microbiome areas of research: a focus on early-life microbial colonization. Microorganisms 2023; 11(2):239. doi: 10.3390/microorganisms11020239 [Crossref] [ Google Scholar]
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev 2012; 70(Suppl 1):S38-44. doi: 10.1111/j.1753-4887.2012.00493.x [Crossref] [ Google Scholar]
- Dickerson F, Dilmore AH, Godoy-Vitorino F, Nguyen TT, Paulus M, Pinto-Tomas AA, et al. The Microbiome and Mental Health Across the Lifespan. Springer; 2022.
- Takayasu L, Watanabe E, Umeyama T, Kurokawa R, Ogata Y, Kiguchi Y, et al. Lifelong temporal dynamics of the gut microbiome associated with longevity in mice. bioRxiv [Preprint]. November 8, 2022. Available from: https://www.biorxiv.org/content/10.1101/2022.11.07.515511v1.
- Woźniak D, Cichy W, Przysławski J, Drzymała-Czyż S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv Med Sci 2021; 66(2):284-92. doi: 10.1016/j.advms.2021.05.003 [Crossref] [ Google Scholar]
- Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Vélez E, Perdigón G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab 2019; 74(2):115-24. doi: 10.1159/000496426 [Crossref] [ Google Scholar]
- Chen Y, Wang H, Lu W, Wu T, Yuan W, Zhu J. Human gut microbiome aging clocks based on taxonomic and functional signatures through multi-view learning. Gut Microbes 2022; 14(1):2025016. doi: 10.1080/19490976.2021.2025016 [Crossref] [ Google Scholar]
- Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev 2017; 279(1):70-89. doi: 10.1111/imr.12567 [Crossref] [ Google Scholar]
- Guo J, Huang X, Dou L, Yan M, Shen T, Tang W. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther 2022; 7(1):391. doi: 10.1038/s41392-022-01251-0 [Crossref] [ Google Scholar]
- Bhatti JS, Khullar N, Vijayvergiya R, Navik U, Bhatti GK, Reddy PH. Mitochondrial miRNA as epigenomic signatures: visualizing aging-associated heart diseases through a new lens. Ageing Res Rev 2023; 86:101882. doi: 10.1016/j.arr.2023.101882 [Crossref] [ Google Scholar]
- Zhao TV, Sato Y, Goronzy JJ, Weyand CM. T-cell aging-associated phenotypes in autoimmune disease. Front Aging 2022; 3:867950. doi: 10.3389/fragi.2022.867950 [Crossref] [ Google Scholar]
- Weinberg J, Gaur M, Swaroop A, Taylor A. Proteostasis in aging-associated ocular disease. Mol Aspects Med 2022; 88:101157. doi: 10.1016/j.mam.2022.101157 [Crossref] [ Google Scholar]
- Gavia-García G, Rosado-Pérez J, Arista-Ugalde TL, Aguiñiga-Sánchez I, Santiago-Osorio E, Mendoza-Núñez VM. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology (Basel) 2021; 10(4):253. doi: 10.3390/biology10040253 [Crossref] [ Google Scholar]
- García-García VA, Alameda JP, Page A, Casanova ML. Role of NF-κB in ageing and age-related diseases: lessons from genetically modified mouse models. Cells 2021; 10(8):1906. doi: 10.3390/cells10081906 [Crossref] [ Google Scholar]
- Remigante A, Morabito R. Cellular and molecular mechanisms in oxidative stress-related diseases. Int J Mol Sci 2022; 23(14):8017. doi: 10.3390/ijms23148017 [Crossref] [ Google Scholar]
- Montazersaheb S, Ehsani A, Fathi E, Farahzadi R. Cellular and molecular mechanisms involved in hematopoietic stem cell aging as a clinical prospect. Oxid Med Cell Longev 2022; 2022:2713483. doi: 10.1155/2022/2713483 [Crossref] [ Google Scholar]
- Zhu X, Chen Z, Shen W, Huang G, Sedivy JM, Wang H. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther 2021; 6(1):245. doi: 10.1038/s41392-021-00646-9 [Crossref] [ Google Scholar]
- Nargeh H, Aliabadi F, Ajami M, Pazoki-Toroudi H. Role of polyphenols on gut microbiota and the ubiquitin-proteasome system in neurodegenerative diseases. J Agric Food Chem 2021; 69(22):6119-44. doi: 10.1021/acs.jafc.1c00923 [Crossref] [ Google Scholar]
- Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 2020; 8(1):36. doi: 10.1186/s40168-020-00821-0 [Crossref] [ Google Scholar]
- Fart F, Rajan SK, Wall R, Rangel I, Ganda-Mall JP, Tingö L. Differences in gut microbiome composition between senior orienteering athletes and community-dwelling older adults. Nutrients 2020; 12(9):2610. doi: 10.3390/nu12092610 [Crossref] [ Google Scholar]
- Shi X, Ma T, Sakandar HA, Menghe B, Sun Z. Gut microbiome and aging nexus and underlying mechanism. Appl Microbiol Biotechnol 2022; 106(17):5349-58. doi: 10.1007/s00253-022-12089-5 [Crossref] [ Google Scholar]
- Dąbrowska K, Witkiewicz W. Correlations of host genetics and gut microbiome composition. Front Microbiol 2016; 7:1357. doi: 10.3389/fmicb.2016.01357 [Crossref] [ Google Scholar]
- Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT. Host genetic variation impacts microbiome composition across human body sites. Genome Biol 2015; 16(1):191. doi: 10.1186/s13059-015-0759-1 [Crossref] [ Google Scholar]
- Bardos J, Fiorentino D, Longman RE, Paidas M. Immunological role of the maternal uterine microbiome in pregnancy: pregnancies pathologies and alterated microbiota. Front Immunol 2019; 10:2823. doi: 10.3389/fimmu.2019.02823 [Crossref] [ Google Scholar]
- De Siena M, Laterza L, Matteo MV, Mignini I, Schepis T, Rizzatti G. Gut and reproductive tract microbiota adaptation during pregnancy: new insights for pregnancy-related complications and therapy. Microorganisms 2021; 9(3):473. doi: 10.3390/microorganisms9030473 [Crossref] [ Google Scholar]
- Avershina E, Storrø O, Øien T, Johnsen R, Pope P, Rudi K. Major faecal microbiota shifts in composition and diversity with age in a geographically restricted cohort of mothers and their children. FEMS Microbiol Ecol 2014; 87(1):280-90. doi: 10.1111/1574-6941.12223 [Crossref] [ Google Scholar]
- Lawley B, Otal A, Moloney-Geany K, Diana A, Houghton L, Heath AM. Fecal microbiotas of Indonesian and New Zealand children differ in complexity and bifidobacterial taxa during the first year of life. Appl Environ Microbiol 2019; 85(19):e01105-19. doi: 10.1128/aem.01105-19 [Crossref] [ Google Scholar]
- Zimmermann P, Curtis N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: a systematic review. Arch Dis Child Fetal Neonatal Ed 2020; 105(2):201-8. doi: 10.1136/archdischild-2018-316659 [Crossref] [ Google Scholar]
- Qian M, Wu N, Li L, Yu W, Ouyang H, Liu X. Effect of elevated ketone body on maternal and infant outcome of pregnant women with abnormal glucose metabolism during pregnancy. Diabetes Metab Syndr Obes 2020; 13:4581-8. doi: 10.2147/dmso.s280851 [Crossref] [ Google Scholar]
- Gupta HD, Choudhury RG. Neonatal disorders and obstetricians. J Indian Med Assoc 2001; 99(5):262-4. [ Google Scholar]
- Satheesh SS, Prasobh GR, Chandran S, Arsha VR. The human microbiota and evolving therapeutic potential. World J Pharm Res 2019; 8(13):689-706. doi: 10.20959/wjpr201913-16314 [Crossref] [ Google Scholar]
- Sohrabi T, Mirzaei-Behbahani B, Zadali R, Pirhaghi M, Morozova-Roche LA, Meratan AA. Common mechanisms underlying α-synuclein-induced mitochondrial dysfunction in Parkinson’s disease. J Mol Biol 2023; 435(12):167992. doi: 10.1016/j.jmb.2023.167992 [Crossref] [ Google Scholar]
- Gerhardt S, Mohajeri MH. Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 2018; 10(6):708. doi: 10.3390/nu10060708 [Crossref] [ Google Scholar]
- Hughes KC, Gao X, Kim IY, Wang M, Weisskopf MG, Schwarzschild MA. Intake of dairy foods and risk of Parkinson disease. Neurology 2017; 89(1):46-52. doi: 10.1212/wnl.0000000000004057 [Crossref] [ Google Scholar]
- Knight E, Geetha T, Burnett D, Babu JR. The role of diet and dietary patterns in Parkinson’s disease. Nutrients 2022; 14(21):4472. doi: 10.3390/nu14214472 [Crossref] [ Google Scholar]
- Marras C, Canning CG, Goldman SM. Environment, lifestyle, and Parkinson’s disease: implications for prevention in the next decade. Mov Disord 2019; 34(6):801-11. doi: 10.1002/mds.27720 [Crossref] [ Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 2017; 32(5):739-49. doi: 10.1002/mds.26942 [Crossref] [ Google Scholar]
- Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife 2020; 9:e53111. doi: 10.7554/eLife.53111 [Crossref] [ Google Scholar]
- Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther 2021; 6(1):291. doi: 10.1038/s41392-021-00687-0 [Crossref] [ Google Scholar]
- Lubomski M, Tan AH, Lim SY, Holmes AJ, Davis RL, Sue CM. Parkinson’s disease and the gastrointestinal microbiome. J Neurol 2020; 267(9):2507-23. doi: 10.1007/s00415-019-09320-1 [Crossref] [ Google Scholar]
- Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H. Colonic inflammation in Parkinson’s disease. Neurobiol Dis 2013; 50:42-8. doi: 10.1016/j.nbd.2012.09.007 [Crossref] [ Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB. Colonic bacterial composition in Parkinson’s disease. Mov Disord 2015; 30(10):1351-60. doi: 10.1002/mds.26307 [Crossref] [ Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 2017; 32(5):739-49. doi: 10.1002/mds.26942 [Crossref] [ Google Scholar]
- Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 2015; 3:12. doi: 10.1186/s40478-015-0196-0 [Crossref] [ Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol 2017; 17(4):219-32. doi: 10.1038/nri.2017.7 [Crossref] [ Google Scholar]
- Dogra N, Mani RJ, Katare DP. The gut-brain axis: two ways signaling in Parkinson’s disease. Cell Mol Neurobiol 2022; 42(2):315-32. doi: 10.1007/s10571-021-01066-7 [Crossref] [ Google Scholar]
- Kaur G, Behl T, Bungau S, Kumar A, Uddin MS, Mehta V. Dysregulation of the gut-brain axis, dysbiosis and influence of numerous factors on gut microbiota associated Parkinson’s disease. Curr Neuropharmacol 2021; 19(2):233-47. doi: 10.2174/1570159x18666200606233050 [Crossref] [ Google Scholar]
- Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis 2020; 135:104352. doi: 10.1016/j.nbd.2018.12.012 [Crossref] [ Google Scholar]
- Chandra S, Sisodia SS, Vassar RJ. The gut microbiome in Alzheimer’s disease: what we know and what remains to be explored. Mol Neurodegener 2023; 18(1):9. doi: 10.1186/s13024-023-00595-7 [Crossref] [ Google Scholar]
- Tarawneh R, Penhos E. The gut microbiome and Alzheimer’s disease: complex and bidirectional interactions. Neurosci Biobehav Rev 2022; 141:104814. doi: 10.1016/j.neubiorev.2022.104814 [Crossref] [ Google Scholar]
- Zhang W, Guo Y, Cheng Y, Yao W, Qian H. Neuroprotective effects of polysaccharide from Sparassiscrispa on Alzheimer’s disease-like mice: involvement of microbiota-gut-brain axis. Int J Biol Macromol 2023; 225:974-86. doi: 10.1016/j.ijbiomac.2022.11.160 [Crossref] [ Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 2017; 7(1):13537. doi: 10.1038/s41598-017-13601-y [Crossref] [ Google Scholar]
- Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 2018; 63(4):1337-46. doi: 10.3233/jad-180176 [Crossref] [ Google Scholar]
- Sun J, Zhang S, Zhang X, Zhang X, Dong H, Qian Y. IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation. J Neuroinflammation 2015; 12:165. doi: 10.1186/s12974-015-0394-5 [Crossref] [ Google Scholar]
- Fairley A, Stewart CJ, Cassidy A, Woodside JV, McEvoy CT. Diet patterns, the gut microbiome, and Alzheimer’s disease. J Alzheimers Dis 2022; 88(3):933-41. doi: 10.3233/jad-220205 [Crossref] [ Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep 2017; 7:41802. doi: 10.1038/srep41802 [Crossref] [ Google Scholar]
- Torchia MT, Amakiri I, Werth P, Moschetti W. Characterization of native knee microorganisms using next-generation sequencing in patients undergoing primary total knee arthroplasty. Knee 2020; 27(3):1113-9. doi: 10.1016/j.knee.2019.12.013 [Crossref] [ Google Scholar]
- Alkady EA, Selim ZI, Abdelaziz MM, El-Hafeez FA. Epidemiology and socioeconomic burden of osteoarthritis. J Curr Med Res Pract 2023; 8(1):7-11. doi: 10.4103/jcmrp.jcmrp_99_19 [Crossref] [ Google Scholar]
- Gleason B, Chisari E, Parvizi J. Osteoarthritis can also start in the gut: the gut-joint axis. Indian J Orthop 2022; 56(7):1150-5. doi: 10.1007/s43465-021-00473-8 [Crossref] [ Google Scholar]
- Kuraji R, Shiba T, Dong TS, Numabe Y, Kapila YL. Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis. World J Gastroenterol 2023; 29(6):967-96. doi: 10.3748/wjg.v29.i6.967 [Crossref] [ Google Scholar]
- Dunn CM, Jeffries MA. The microbiome in osteoarthritis: a narrative review of recent human and animal model literature. Curr Rheumatol Rep 2022; 24(5):139-48. doi: 10.1007/s11926-022-01066-6 [Crossref] [ Google Scholar]
- Arbeeva L, Azcarate-Peril MA, Cui Y, Nelson AE, Loeser RF. Association of plasma microbial composition with a leaky gut in obesity-related osteoarthritis: an exploratory study. Osteoarthr Cartil Open 2022; 4(4):100317. doi: 10.1016/j.ocarto.2022.100317 [Crossref] [ Google Scholar]
- O-Sullivan I, Natarajan Anbazhagan A, Singh G, Ma K, Green SJ, Singhal M. Lactobacillus acidophilus mitigates osteoarthritis-associated pain, cartilage disintegration and gut microbiota dysbiosis in an experimental murine OA model. Biomedicines 2022; 10(6):1298. doi: 10.3390/biomedicines10061298 [Crossref] [ Google Scholar]
- Javier-DesLoges J, McKay RR, Swafford AD, Sepich-Poore GD, Knight R, Parsons JK. The microbiome and prostate cancer. Prostate Cancer Prostatic Dis 2022; 25(2):159-64. doi: 10.1038/s41391-021-00413-5 [Crossref] [ Google Scholar]
- Maffei S, Forini F, Canale P, Nicolini G, Guiducci L. Gut microbiota and sex hormones: crosstalking players in cardiometabolic and cardiovascular disease. Int J Mol Sci 2022; 23(13):7154. doi: 10.3390/ijms23137154 [Crossref] [ Google Scholar]
- Giambartolomei C, Seo JH, Schwarz T, Freund MK, Johnson RD, Spisak S. H3K27ac HiChIP in prostate cell lines identifies risk genes for prostate cancer susceptibility. Am J Hum Genet 2021; 108(12):2284-300. doi: 10.1016/j.ajhg.2021.11.007 [Crossref] [ Google Scholar]
- Fujita K, Matsushita M, Banno E, De Velasco MA, Hatano K, Nonomura N. Gut microbiome and prostate cancer. Int J Urol 2022; 29(8):793-8. doi: 10.1111/iju.14894 [Crossref] [ Google Scholar]
- Golombos DM, Ayangbesan A, O’Malley P, Lewicki P, Barlow L, Barbieri CE. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology 2018; 111:122-8. doi: 10.1016/j.urology.2017.08.039 [Crossref] [ Google Scholar]
- Matsushita M, Fujita K, Motooka D, Hatano K, Fukae S, Kawamura N. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci 2021; 112(8):3125-35. doi: 10.1111/cas.14998 [Crossref] [ Google Scholar]
- Liss MA, White JR, Goros M, Gelfond J, Leach R, Johnson-Pais T. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur Urol 2018; 74(5):575-82. doi: 10.1016/j.eururo.2018.06.033 [Crossref] [ Google Scholar]
- Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res 2021; 81(15):4014-26. doi: 10.1158/0008-5472.can-20-4090 [Crossref] [ Google Scholar]
- Ahmad Kendong SM, Raja Ali RA, Nawawi KNM, Ahmad HF, Mokhtar NM. Gut dysbiosis and intestinal barrier dysfunction: potential explanation for early-onset colorectal cancer. Front Cell Infect Microbiol 2021; 11:744606. doi: 10.3389/fcimb.2021.744606 [Crossref] [ Google Scholar]
- Hisada H, Takahashi Y, Kubota M, Shimura H, Itobayashi E, Shimura K. Clinical and therapeutic features and prognostic factors of metastatic colorectal cancer over age 80: a retrospective study. BMC Gastroenterol 2021; 21(1):199. doi: 10.1186/s12876-021-01791-9 [Crossref] [ Google Scholar]
- Ali Khan U, Fallah M, Sundquist K, Sundquist J, Brenner H, Kharazmi E. Risk of colorectal cancer in patients with diabetes mellitus: a Swedish nationwide cohort study. PLoS Med 2020; 17(11):e1003431. doi: 10.1371/journal.pmed.1003431 [Crossref] [ Google Scholar]
- Lavoie S, Garrett WS. The unfolding story of ATF6, microbial dysbiosis, and colorectal cancer. Gastroenterology 2018; 155(5):1309-11. doi: 10.1053/j.gastro.2018.10.011 [Crossref] [ Google Scholar]
- Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K. Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS One 2017; 12(11):e0187307. doi: 10.1371/journal.pone.0187307 [Crossref] [ Google Scholar]