Int J Aging.2023;1:e21.
doi: 10.34172/ija.2023.e21
Review Article
Anti-amyloid Monoclonal Antibodies in Alzheimer’s Disease: A New Hope
Aygun Nasibova 1  , Rovshan Khalilov 2, Aziz Eftekhari 3, *
, Rovshan Khalilov 2, Aziz Eftekhari 3, * 
Author information:
1Institute of Radiation Problems, Azerbaijan National Academy of Sciences, Baku, Azerbaijan
2Baku State University, Baku, Azerbaijan
3Department of Toxicology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Objectives:
The current review aimed to collect the updated clinical investigations of monoclonal antibodies (mAbs) in Alzheimer’s disease (AD) patients and assess the feasibility and efficacy of the immunotherapies with mAbs in AD.
Design:
A narrative review.
Participants:
People with AD.
Outcome measures:
The occurrence of AD.
Results:
The failure of all attempts to cure AD during the previous decades by focusing on the pathogenic factors revealed that the pathophysiology of AD is multifaceted. In recent years, anti-amyloid mAbs that exhibit beneficial effects in AD treatment have been marketed, and different studies have attempted to assess their effects. We investigated the current research to determine the potential benefits and outcomes of clinical trials of mAbs in AD cases in the review.
Conclusions:
This study concentrated on determining how these drugs affect AD pathology. It offers potential support for using anti-amyloid mAb immunotherapy for AD and evaluates the lessons learned from these clinical studies to conduct further research on the beneficial and harmful impacts of these therapeutic agents.
Keywords: Anti-amyloid, Monoclonal antibodies, Alzheimer’s disease
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
As a chronic neurodegenerative ailment, Alzheimer’s disease (AD) is characterized by cognition impairment, loss of memory, and disruption of synaptic pathways.1,2 Over one hundred million individuals on the planet by the year 2050, and 10% to 30% of those who are 65 or above will have AD.3 The disease has a considerable influence on the economy as a global public health crisis.2,4 The main primary hallmarks of the disease are neurofibrillary tangles and amyloid plaques caused by intracellular tau protein hyperphosphorylation.5 The amyloidogenic cascade and its peptide byproducts play a significant role in the start of AD.6 These peptides can accumulate in brain tissue and vasculature, leading to neuronal atrophy.7 Furthermore, AD development may potentially be influenced by inflammation.8 Scanty medicines, including non-competitive N-methyl-D-aspartate receptor antagonists and acetylcholinesterase blockers, have been used clinically to treat AD to date.9 Nevertheless, these medications can only partially relieve symptoms, and they cannot stop the course of AD. According to the amyloid cascade theory, removing brain plaques may treat AD and halt the progression of the illness. This idea has sparked the creation of cutting-edge medications to stop amyloid beta aggregation in neural tissue in recent years. However, earlier attempts to cure AD by focusing on pathogenic amyloid or tau have all failed, suggesting that the disease’s pathophysiology is more complicated and multifaceted.10 Anti-amyloid treatments are currently being discussed. Although the neural system has traditionally been regarded as an immune-privileged location, there are mounting reports suggesting that innate immunity plays a significant role in AD’s pathogenesis.11 Additionally, recent discoveries regarding the pathogenesis and treatment of AD12 revealed that the brain contains accumulated amyloid and misfolded tau proteins which led to adaptive immune response. These discoveries may open up new treatment options for this disease, including active and passive anti-amyloid immunotherapies that clear brain amyloid deposits.13 Aducanumab, lecanemab, gantenerumab, solanezumab, and bapineuzumab are only a few anti-amyloid mAbs being researched as a possible treatment for AD.13 These mAbs distinguish epitopes according to the precise portion and conformations of amyloid, and they differ in their selectivity for polymorphic variants.14 To reduce the amount and toxicity of amyloid in the brain, mAbs target amyloid through the different metabolic cascades, removing or preventing the misfolded amyloid.13 These mAbs identify epitopes according to the amyloid, and they differ in their selectivity for polymorphic variants.13 Clinical trial results to date seem to support the amyloid theory in the development of AD. However, there were numerous randomized control trial failures in the use of mAbs, leaving open the question of how amyloid-targeting medications may be developed in the future. This study collected the updated clinical investigations of mAbs in AD patients and then assessed the feasibility and efficacy of the immunotherapies with mAbs in AD.
Methods
We used Google Scholar, PubMed, and ClinicalTrials.gov on June 15, 2023, with the combinations of the succeeding keywords such as “monoclonal antibody”, “Solanezumab”, “Aducanumab”, “Alzheimer’s”, “Lecanemab”, “BIIB037”, “sporadic”, “mild cognitive impairment”, and “passive immunotherapy”.
Results
By injecting amyloid antigens (active immunization) or anti-amyloid antibodies into the brain of AD patients, immunotherapeutic methods help amyloid be cleared from the brain of AD.13 Passive anti-amyloid antibody vaccination can improve the clearance of amyloid from the brain and plasma, resulting in a reduction in amyloid burden.15
Late-phase trials with most mAbs have yielded disappointing results so far. Notwithstanding the dispute, aducanumab’s phase III research indicated somewhat encouraging results,16 which supports the idea of continuing to explore the anti-amyloid mAbs for the management of AD. Some of the drugs described in this study that help lower brain amyloid levels are still being tested in clinical studies.17
Unlike monomers or insoluble fibrils, soluble amyloid-beta protofibrils have been found to be more hazardous to neurons, and lecanemab is a humanized monoclonal antibody that attaches to them with high affinity.18 In a Bayesian analysis of the 12-month alteration in score from a dose-finding phase 2b trial containing 854 individuals with initial AD, lecanemab, and placebo failed to significantly differ from each other (primary endpoint).19 Lecanemab, however, was associated with less clinical decline than placebo on several measures, and investigations on 1.5-year-old individuals demonstrated time/dose-dependent amyloid clearance with the medication.20 Lecanemab was found to be effective when prescribed intravenously (10 mg/kg every two weeks) of amyloid-related imaging abnormalities (ARIAs).21 Moreover, lecanemab was tested for safety and effectiveness in patients with early AD as part of a phase 3 experiment known as clarity AD.22 Lecanemab decreased amyloid indicators in early AD, moderately slowed cognitive and functional decline compared to placebo after 18 months; however, it was also led to negative side effects.23 Accordingly, lecanemab’s efficiency and safety in the treatment of early AD call for longer trials.
Via peptide aggregation separation and fibrillar amyloid clearance, the completely human amyloid immunoglobulin G1 antibody gantenerumab is intended to improve the clearance of amyloid plaques in the neural tissue.24 The human immunoglobulin G1 backbone stimulates microglial phagocytosis of aggregated amyloid via the Fc gamma receptor.25 Gantenerumab’s capability to attach to amyloid in ex vivo plaques was demonstrated by electron microscopy, and binding to amyloid in the brains of AD patients was exhibited by immunofluorescence staining.25 Postmortem analysis of AD brain tissue reveals that phagocytosis by the Fc gamma receptor and microglia, after degradation of lysosomes, removes fluorescent-labeled gantenerumab linked to amyloid plaques.26 The affinity of gantenerumab is highest for aggregated forms of amyloid and soluble oligomers. According to preliminary findings, binding to oligomers takes place and reduces their toxicity.27 According to the above-mentioned evidence, gantenerumab inhibits the growth of aggregated amyloid and activates microglial phagocytosis, exerting two significant impacts on aggregated amyloid.25 The effect of gantenerumab AD pathology and neurodegeneration is on amyloid, comprising time/dose -dependant diminutions in total tau levels in cerebrospinal fluid phosphorylated tau and reducing the neurofilament light chain and neurogranin28,29 (Figure 1).
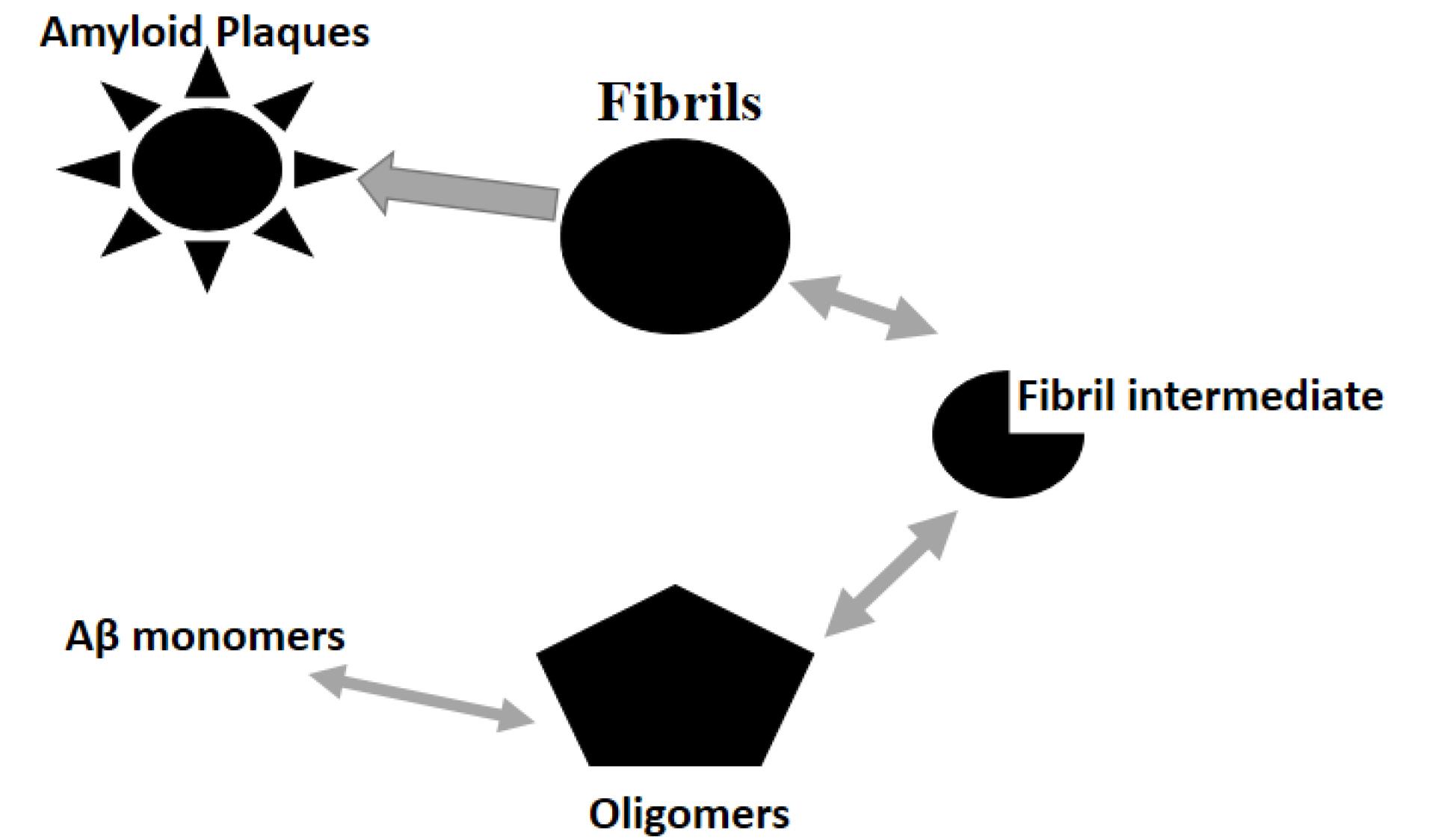
Figure 1.
The Amyloid Theory Includes the Aβ Soluble Monomer/Oligomer Aggregation in Insoluble Amyloid Plaques and Fibrils
.
The Amyloid Theory Includes the Aβ Soluble Monomer/Oligomer Aggregation in Insoluble Amyloid Plaques and Fibrils
Human immunoglobulin 1 (IgG1) mAb aducanumab can only target aggregated amyloid such as neuritic amyloid plaques and high molecular weight, but it does not target amyloid monomers.13 Furthermore, aducanumab exhibits a preference for parenchymal versus vascular amyloid in the brain.30 Anti-amyloid mAb therapies in AD in vivo models have been highly effective in reducing brain damage and enhancing cognitive abilities through microglial activation and amyloid aggregation prevention.31 It has been demonstrated that aducanumab has a definite therapeutic effect and completely eradicates amyloid from mouse brain tissue. Transgenic (tg) mice’s brains can absorb aducanumab, which binds to parenchymal amyloid and inhibits both soluble and insoluble amyloids.32 Old tgAPPPS1-21 mice (an experimental model for AD) were given weekly doses of aducanumab (10 mg/kg) for four months, and the results demonstrated that aducanumab clearly reduces amyloid cytotoxicity and improves phagocytosis and cell survival.13
The injection of aducanumab combined with an ultrasound animal model of AD can improve cognition because ultrasound scans with intravenously administered microbubbles provisionally breach the blood-brain barrier, allowing aducanumab’s access to the brain and increasing brain levels of aducanumab.33 Aducanumab could reduce amyloid plaque in a concentration-dependent way in Tg2576 mice as an AD model but not in 22-month-old animals, showing that it was more successful at preventing amyloid aggregation than at removing the pre-existing amyloid plaques.30,34 However, after receiving aducanumab medication, these mice did not exhibit any improvement in their cognition or behavior.30
The US Food and Drug Administration (FDA) authorized aducanumab as the first disease-modifying therapy for AD in June 2021.35 Mild dementia and cognitive impairment were treated with aducanumab, and the positive advance in the synthesis of aducanumab is regarded as groundbreaking progress in the treatment of AD.36 Aducanumab slows cognitive decline in mild or prodromal AD as judged by the Mini-Mental State Examination (MMSE) and Clinical Dementia Rating-Sum of Boxes (CDR-SB) scores by binding to amyloid plaques and the amyloid-β oligomer and inducing amyloid clearance.13
Discussion
Aducanumab medication produced positive results in a phase Ib double-blind, randomized, and placebo-controlled study, and it was found to be beneficial for AD-related mild cognitive impairment (MCI) or mild AD dementia.13 As a result, phase III clinical studies for aducanumab were initiated in 2015.37 According to a study containing 196 patients with AD, amyloid plaques reduced and gradually decreased in clinical parameters in moderate or prodromal AD using aducanumab.13 Aducanumab also showed considerable efficacy on clinical and biomarker results, a favorable safety and tolerability outline.38 The negative effect of removing amyloid, known as ARIAs, was dose-dependent in the group receiving aducanumab. Being the most solid genetic risk factor for delayed AD, APOE4 carriers experienced ARIAs more frequently than non-carriers,39 which was a key safety result and should support the additional use of aducanumab for AD treatment.40 Recent notable findings from a phase III trial using aducanumab showed the drug’s small but highest efficacy, providing crucial amyloid confirmation as a therapeutic target.41
In phase Ib, IV, and III studies, aducanumab has been found to be an effective treatment for reducing amyloid plaques which is likely related to the dose and length of treatment.42 The investigations of 803 patients (Emerge) and 945 patients (Engage) in the 2018 trial were different, and the results were not always consistent.13 These studies revealed that aducanumab was applied positively in the Emerge group and negatively in the Engage group.13,43
Fortunately, several follow-up studies involving the Emerge and Engage groups revealed that aducanumab-treated individuals in the Engage group have a modest drop in the CDR-SB parallel to the Emerge group alterations (Table 1).13 Although inconsistent results were observed in these investigations regarding cognitive outcomes, the trials demonstrated exceptional dose-dependent amyloid elimination in both treatments (Table 1).13
Table 1.
Clinical Trials of a Immunotherapy for the Treatment of AD
|
Type of Agent
|
Period
|
Main Efficiency Measures
|
Other Results
|
References
|
| Bapineuzumab, |
78 |
DAD ADASCog11 |
DS, NTB, CDR-SB |
Vandenberghe et al44 |
| Aducanumab, |
78 |
CDR-SB |
ADCS-ADL-MC, MMSE, ADAS-Cog |
Emerge study45 |
| Aducanumab, |
78 |
CDR-SB |
ADCS-ADL-MCI, MMSE, ADAS-Cog |
Emerge study 45 |
| Aducanumab, |
78 |
CDR-SB |
ADCS-ADL-MCI, MMSE, ADAS-Cog |
Engage study45 |
| Aducanumab, |
78 |
CDR-SB |
ADCS-ADL-MC, MMSE, ADAS-Cog |
Engage study 45 |
| Crenezumab |
100 |
CDR-SB |
ADCS- iADL, ADCS-ADL, ADAS-Cog, CDR-GS, MMSE |
CREAD1 study 46 |
| Crenezumab |
100 |
CDR-SB |
iADL, ADCS- ADCS-ADL, ADAS-Cog, CDR-GS, MMSE |
CREAD2 study 47 |
Note. A: Amyloid; AD: Alzheimer’s disease; DAD: Disability assessment for dementia; ADAS-Cog: Alzheimer’s disease assessment scale-Cognitive subscale; DS: Dependence scale; NTB: Neuropsychological test battery; CDR-SB: Clinical dementia rating-Sum of boxes; ADCS-ADL: Alzheimer’s Disease Cooperative Study– Activities of Daily Living; MCI: Mild cognitive impairment; MMSE: Mini-mental state examination; CDR-GS: Clinical dementia rate-Global score; FAQ: Functional activities questionnaire; MMSE: Mini-mental state examination.
Conclusions
Since 2005, 101 trials examining mAbs in cases with MCI or AD have been registered, and data are accessible from 50 trials comprising about 18 000 people.48 The results of a recent meta-analysis revealed that there is still uncertainty regarding the risk-benefit sketch of mAbs. Clarifying how amyloid affects cognitive decline, supplying information on treatment response rates, and accounting for small clinically significant differences should be the main goals of future research.
Clarifying whether eliminating the amyloid burden has an impact on the development of cognitive decline should be the main goal of mAb research, along with providing information on treatment response rates that take the minimal clinically important difference into account. This is especially important given that the target demographic is trending toward those who are at an earlier stage of the disease on the premise that eliminating plaques from a brain that is still largely intact can have a greater clinical impact. Since the implementation of mAbs is still connected with a significantly increased risk of ARIA, even in the most recently developed ones, research on these medications should also concentrate on evaluating the potential long-term effects of ARIA events and looking into potential elements envisaging their commencement.
Funding
None.
Data availability statement
Not applicable.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that there is no conflict of interests.
References
- Zandi PP, Jaffe AE, Goes FS, Burke EE, Collado-Torres L, Huuki-Myers L. Amygdala and anterior cingulate transcriptomes from individuals with bipolar disorder reveal downregulated neuroimmune and synaptic pathways. Nat Neurosci 2022; 25(3):381-9. doi: 10.1038/s41593-022-01024-6 [Crossref] [ Google Scholar]
- Safiri S, Noori M, Nejadghaderi SA, Mousavi SE, Sullman MJM, Araj-Khodaei M. The burden of Alzheimer’s disease and other types of dementia in the Middle East and North Africa region, 1990-2019. Age Ageing 2023; 52(3):afad042. doi: 10.1093/ageing/afad042 [Crossref] [ Google Scholar]
- Rayathala J, Kumar K, Venkatesh P. Review on Alzheimer’s disease: past, present and future. J Innov Appl Pharm Sci 2022; 7(1):28-31. doi: 10.37022/jiaps.v7i1.274 [Crossref] [ Google Scholar]
- Kam S, Hwang BJ, Parker ER. The impact of climate change on atopic dermatitis and mental health comorbidities: a review of the literature and examination of intersectionality. Int J Dermatol 2023; 62(4):449-58. doi: 10.1111/ijd.16557 [Crossref] [ Google Scholar]
- Tabatabaei Mohammadi A, Bahaeddini M, Dehghani N, Dehghani N, Shafagh SG, Qahremani R, et al. Alzheimer’s Disease: For Researchers: Neuropathology, Biochemistry, Disease Mechanism. Nobel TM; 2022.
- Nasb M, Tao W, Chen N. Alzheimer’s disease puzzle: delving into pathogenesis hypotheses. Aging Dis 2024; 15(1):1-31. doi: 10.14336/ad.2023.0608 [Crossref] [ Google Scholar]
- Maitre M, Jeltsch-David H, Okechukwu NG, Klein C, Patte-Mensah C, Mensah-Nyagan AG. Myelin in Alzheimer’s disease: culprit or bystander?. Acta Neuropathol Commun 2023; 11(1):56. doi: 10.1186/s40478-023-01554-5 [Crossref] [ Google Scholar]
- Novoa C, Salazar P, Cisternas P, Gherardelli C, Vera-Salazar R, Zolezzi JM. Inflammation context in Alzheimer’s disease, a relationship intricate to define. Biol Res 2022; 55(1):39. doi: 10.1186/s40659-022-00404-3 [Crossref] [ Google Scholar]
- Argueta N, Notari E, Szigeti K. Role of pharmacogenomics in individualizing treatment for Alzheimer’s disease. CNS Drugs 2022; 36(4):365-76. doi: 10.1007/s40263-022-00915-3 [Crossref] [ Google Scholar]
- Li T, Lu L, Pember E, Li X, Zhang B, Zhu Z. New insights into neuroinflammation involved in pathogenic mechanism of Alzheimer’s disease and its potential for therapeutic intervention. Cells 2022; 11(12):1925. doi: 10.3390/cells11121925 [Crossref] [ Google Scholar]
- Zang X, Chen S, Zhu J, Ma J, Zhai Y. The emerging role of central and peripheral immune systems in neurodegenerative diseases. Front Aging Neurosci 2022; 14:872134. doi: 10.3389/fnagi.2022.872134 [Crossref] [ Google Scholar]
- Chen X, Holtzman DM. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity 2022; 55(12):2236-54. doi: 10.1016/j.immuni.2022.10.016 [Crossref] [ Google Scholar]
- Shi M, Chu F, Zhu F, Zhu J. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: a focus on aducanumab and lecanemab. Front Aging Neurosci 2022; 14:870517. doi: 10.3389/fnagi.2022.870517 [Crossref] [ Google Scholar]
- Lloyd GM, Sorrentino ZA, Quintin S, Gorion KM, Bell BM, Paterno G. Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice. Acta Neuropathol 2022; 143(6):663-85. doi: 10.1007/s00401-022-02425-4 [Crossref] [ Google Scholar]
- Alshamrani M. Recent trends in active and passive immunotherapies of Alzheimer’s disease. Antibodies (Basel) 2023; 12(2):41. doi: 10.3390/antib12020041 [Crossref] [ Google Scholar]
- Zhu Y. The current status of drugs related to amyloid β-protein in Alzheimer’s disease. Highlights in Science, Engineering and Technology 2023; 36:762-7. doi: 10.54097/hset.v36i.5792 [Crossref] [ Google Scholar]
- Alves F, Kalinowski P, Ayton S. Accelerated brain volume loss caused by anti-β-amyloid drugs: a systematic review and meta-analysis. Neurology 2023; 100(20):e2114-e24. doi: 10.1212/wnl.0000000000207156 [Crossref] [ Google Scholar]
- Lazarev VF, Dutysheva EA, Kanunikov IE, Guzhova IV, Margulis BA. Protein interactome of amyloid-β as a therapeutic target. Pharmaceuticals (Basel) 2023; 16(2):312. doi: 10.3390/ph16020312 [Crossref] [ Google Scholar]
- Swanson C, Dhadda S, Hodgkinson M, Li D, Kanekiyo M, Kaplow J. Lecanemab, an anti-Aβ protofibril antibody: updated data from a randomized, double-blind phase 2B proof of concept clinical trial and open-label extension in early Alzheimer’s disease. J Neurol Sci 2021; 429:117847. doi: 10.1016/j.jns.2021.117847 [Crossref] [ Google Scholar]
- McDade E, Cummings JL, Dhadda S, Swanson CJ, Reyderman L, Kanekiyo M. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther 2022; 14(1):191. doi: 10.1186/s13195-022-01124-2 [Crossref] [ Google Scholar]
- Liu KY, Villain N, Ayton S, Ackley SF, Planche V, Howard R. Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer’s disease. Brain Commun 2023; 5(3):fcad175. doi: 10.1093/braincomms/fcad175 [Crossref] [ Google Scholar]
- Vitek GE, Decourt B, Sabbagh MN. Lecanemab (BAN2401): an anti-beta-amyloid monoclonal antibody for the treatment of Alzheimer disease. Expert Opin Investig Drugs 2023; 32(2):89-94. doi: 10.1080/13543784.2023.2178414 [Crossref] [ Google Scholar]
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M. Lecanemab in early Alzheimer’s disease. N Engl J Med 2023; 388(1):9-21. doi: 10.1056/NEJMoa2212948 [Crossref] [ Google Scholar]
- Liu Y, Cong L, Han C, Li B, Dai R. Recent progress in the drug development for the treatment of Alzheimer’s disease especially on inhibition of amyloid-peptide aggregation. Mini Rev Med Chem 2021; 21(8):969-90. doi: 10.2174/1389557520666201127104539 [Crossref] [ Google Scholar]
- Bateman RJ, Cummings J, Schobel S, Salloway S, Vellas B, Boada M. Gantenerumab: an anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimers Res Ther 2022; 14(1):178. doi: 10.1186/s13195-022-01110-8 [Crossref] [ Google Scholar]
- Jäntti H, Sitnikova V, Ishchenko Y, Shakirzyanova A, Giudice L, Ugidos IF. Microglial amyloid beta clearance is driven by PIEZO1 channels. J Neuroinflammation 2022; 19(1):147. doi: 10.1186/s12974-022-02486-y [Crossref] [ Google Scholar]
- Wu Y, Guo S, Wang K, Kang J. The interaction of peptide inhibitors and Aβ protein: binding mode analysis, inhibition of the formation of Aβ aggregates, and then exert neuroprotective effects. Front Aging Neurosci 2023; 15:1139418. doi: 10.3389/fnagi.2023.1139418 [Crossref] [ Google Scholar]
- Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther 2017; 9(1):95. doi: 10.1186/s13195-017-0318-y [Crossref] [ Google Scholar]
- Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med 2021; 27(7):1187-96. doi: 10.1038/s41591-021-01369-8 [Crossref] [ Google Scholar]
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016; 537(7618):50-6. doi: 10.1038/nature19323 [Crossref] [ Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 2013; 80(2):402-14. doi: 10.1016/j.neuron.2013.07.046 [Crossref] [ Google Scholar]
- Punyakoti P, Behl T, Sehgal A, Yadav S, Sachdeva M, Anwer MK. Postulating the possible cellular signalling mechanisms of antibody drug conjugates in Alzheimer’s disease. Cell Signal 2023; 102:110539. doi: 10.1016/j.cellsig.2022.110539 [Crossref] [ Google Scholar]
- Leinenga G, Koh WK, Götz J. A comparative study of the effects of aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease. Alzheimers Res Ther 2021; 13(1):76. doi: 10.1186/s13195-021-00809-4 [Crossref] [ Google Scholar]
- Gamage KK, Kumar S. Aducanumab therapy ameliorates calcium overload in a mouse model of Alzheimer’s disease. J Neurosci 2017; 37(17):4430-2. doi: 10.1523/jneurosci.0420-17.2017 [Crossref] [ Google Scholar]
- Food and Drug Administration (FDA). FDA Authorizes First Interoperable, Automated Insulin Dosing Controller Designed to Allow More Choices for Patients Looking to Customize Their Individual Diabetes Management Device System. FDA; 2021.
- Decourt B, Noorda K, Noorda K, Shi J, Sabbagh MN. Review of advanced drug trials focusing on the reduction of brain beta-amyloid to prevent and treat dementia. J Exp Pharmacol 2022; 14:331-52. doi: 10.2147/jep.s265626 [Crossref] [ Google Scholar]
- Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis 2022; 9(2):197-210. doi: 10.14283/jpad.2022.30 [Crossref] [ Google Scholar]
- Ferrero J, Williams L, Stella H, Leitermann K, Mikulskis A, O’Gorman J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement (N Y) 2016; 2(3):169-76. doi: 10.1016/j.trci.2016.06.002 [Crossref] [ Google Scholar]
- Khoury R, Gallop A, Roberts K, Grysman N, Lu J, Grossberg GT. Pharmacotherapy for Alzheimer’s disease: what’s new on the horizon?. Expert Opin Pharmacother 2022; 23(11):1305-23. doi: 10.1080/14656566.2022.2097868 [Crossref] [ Google Scholar]
- Se Thoe E, Fauzi A, Tang YQ, Chamyuang S, Chia AYY. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci 2021; 276:119129. doi: 10.1016/j.lfs.2021.119129 [Crossref] [ Google Scholar]
- Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement 2020; 16(11):1553-60. doi: 10.1016/j.jalz.2019.09.075 [Crossref] [ Google Scholar]
- Budd Haeberlein S, O’Gorman J, Chiao P, Bussière T, von Rosenstiel P, Tian Y. Clinical development of aducanumab, an anti-Aβ human monoclonal antibody being investigated for the treatment of early Alzheimer’s disease. J Prev Alzheimers Dis 2017; 4(4):255-63. doi: 10.14283/jpad.2017.39 [Crossref] [ Google Scholar]
- Knopman DS, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimers Dement 2021; 17(4):696-701. doi: 10.1002/alz.12213 [Crossref] [ Google Scholar]
- Vandenberghe R, Rinne JO, Boada M, Katayama S, Scheltens P, Vellas B. Bapineuzumab for mild to moderate Alzheimer’s disease in two global, randomized, phase 3 trials. Alzheimers Res Ther 2016; 8(1):18. doi: 10.1186/s13195-016-0189-7 [Crossref] [ Google Scholar]
- Budd Haeberlein S, von Hehn C, Tian Y, Chalkias S, Muralidharan KK, Chen T. EMERGE and ENGAGE topline results: phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimers Dement (N Y) 2020; 16(S9):e047259. doi: 10.1002/alz.047259 [Crossref] [ Google Scholar]
- Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev 2021; 68:101339. doi: 10.1016/j.arr.2021.101339 [Crossref] [ Google Scholar]
- Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS. Evaluating the safety and efficacy of crenezumab vs placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol 2022; 79(11):1113-21. doi: 10.1001/jamaneurol.2022.2909 [Crossref] [ Google Scholar]
- Lacorte E, Ancidoni A, Zaccaria V, Remoli G, Tariciotti L, Bellomo G. Safety and efficacy of monoclonal antibodies for Alzheimer’s disease: a systematic review and meta-analysis of published and unpublished clinical trials. J Alzheimers Dis 2022; 87(1):101-29. doi: 10.3233/jad-220046 [Crossref] [ Google Scholar]