Int J Aging.2023;1 :e10.
doi: 10.34172/ija.2023.e10
Original Article
The Predictive Role of Age and the Systemic Inflammation Indexes on ICU Admission and Mortality in Patients with COVID-19
Peiman Foroughi 1  , Mojtaba Varshochi 2
, Mojtaba Varshochi 2  , Mehdi Hassanpour 3
, Mehdi Hassanpour 3  , Behnam Amini 4, 5
, Behnam Amini 4, 5  , Zeinab Nikniaz 6, *
, Zeinab Nikniaz 6, *  , Hassan Amini 4, 7, *
, Hassan Amini 4, 7, * 
Author information:
1Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Clinical Biochemistry and Laboratory Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
5School of Management and Medical Informatics, Tabriz University of Medical Sciences, Tabriz, Iran
6Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
7Department of General Surgery, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Objectives:
This study was conducted to collect evidence that specified the role of demographic findings (emphasizing age) and systemic inflammatory indicators in the coronavirus disease 2019 (COVID-19) and the outcome of disease which could help clinicians to predict mortality and intensive care unit (ICU) admission of COVID-19 patients.
Design:
A retrospective cohort study.
Setting(s):
Tabriz, the capital city of the East Azerbaijan Province in northwestern Iran.
Participants:
This retrospective cohort study involved analyzing the medical records of 311 COVID-19 patients from 22 July, 2020 to 22 August, 2020.
Outcome measures:
The demographic, clinical, and laboratory data and outcomes such as death, ICU admission, and discharge were extracted from medical records and electronic case records.
Results:
The analysis of collected data revealed that the average age of non-survivor patients was 68.53±14.68 which was significantly higher than that of survivor patients (59.30±16.44). Furthermore, the comparison of data showed that ischemic heart disease (IHD), respiratory diseases, hemoglobin, derived neutrophil-to-lymphocyte ratio (dNLR), NLR, platelet-to-lymphocyte ratio (PLR), and lactate dehydrogenase (LDH) were higher in non-survivors and ICU admitted patients than in survivors and non-ICU admitted patients. Moreover, multivariate logistic regression analysis indicated that only hypertension (Odds ratio [OR]: 3.18, P=0.02) is an independent risk factor of death in COVID-19 patients, and PLR (OR: 1.02, P=0.05), hypertension (OR: 4.00, P=0.002), and IHD (OR: 5.15, P=0.008) were independent risk factor of ICU admission in COVID-19 patients.
Conclusions:
Elderly patients were at higher risk of death and ICU admission compared to others. Further, demographic characteristics and systemic inflammatory indicators were valuable factors for predicting mortality and ICU admission of COVID-19 patients. Collective data regarding the role of demographic characteristics and systemic inflammation indicators for predicting disease outcomes provide strong evidence for the clinical use of these indicators prospectively.
Keywords: COVID-19, Aged patient, Blood cell count, Mortality, Intensive care unit, Patient outcome assessment
Introduction
The coronavirus disease 2019 (COVID-19) shortly spread around the world, and due to the considerable impact of COVID-19 on people’s health worldwide, the World Health Organization (WHO) declared it a pandemic on 11 March, 2020.1-3 Although all ages are susceptible to being infected with COVID-19, older patients with underlying diseases were predisposed to several conditions of comorbidity such as severe acute respiratory distress due to diffuse alveolar damage with cellular fibro myxoid exudates.4 However, most patients are asymptomatic or have mild upper respiratory tract symptoms. COVID-19 in some cases caused pneumonia that can be severe and characterized by dyspnea, fever, cough, pulmonary edema, bilateral pulmonary infiltration, and acute respiratory distress syndrome requiring comprehensive care.5 The restriction of hospital facilities such as mechanical ventilators and intensive care unit (ICU) beds and the presence of new mutations in the novel coronavirus that may increase its pathogenicity have made COVID-19 be considered a life-treating disease so far. Therefore, it is critical to determine the risk factors present in the poor prognosis and predictive indicators of a severe condition.
Since the outbreak, several studies have been conducted to identify predictive factors associated with poor prognosis of severe acute respiratory syndrome coronavirus 2 and reported some indicators such as older age, underlying disease (e.g., diabetes, cardiovascular disease, and hypertension), gender, C-reactive protein, interleukin-6, and D-dimer.1,6-8 Older age was identified as a main risk factor for the severity of COVID-19 symptoms and signs and even as a risk factor for mortality.9 The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been recently examined as prognostic indicators, and it has been discovered that their increased levels might lead to a poor prognosis in different diseases such as infectious disease, malignant tumors, and inflammatory disorders.10-17 Recent investigations suggested that NLR and PLR are valuable predictive factors for the severity and prognosis of COVID-19 and the mortality of patients.18 However, further investigations with a larger population are needed to explore and confirm the levels of NLR and PLR in predicting the hospital stay days, need for ICU, ICU stay days, complementary interventions (e.g., mechanical ventilation and the like), and severity and mortality of COVID-19. In this study, we aimed to investigate whether the NLR and PLR during hospitalization can help health providers to predict the mortality rate and ICU admission of hospitalized patients with COVID-19.
Methods
Patient Selection and Study Design
An initial sample of 311 consecutive patients’ medical records with COVID-19 diagnosis from 22 July, 2020 to 22 August, 2020 were screened for possible inclusion in the study. All the laboratory-confirmed patients were admitted to Imam Reza and Sina Hospital in Tabriz (the referral hospitals in the northwestern region of Iran).
The patients with hematologic disorders and any active condition that could influence the blood cell count were excluded. The exclusion criteria were patients who received preoperative chemotherapy/radiotherapy (n = 8), patients with hematological or autoimmune disease (n = 12), and the lack of complete medical records (n = 41). Totally, 61 patients were excluded. The enrollment process is presented in Figure 1. Only the laboratory-confirmed patients (based on the diagnostic criteria of WHO guidance) were enrolled in the analysis.
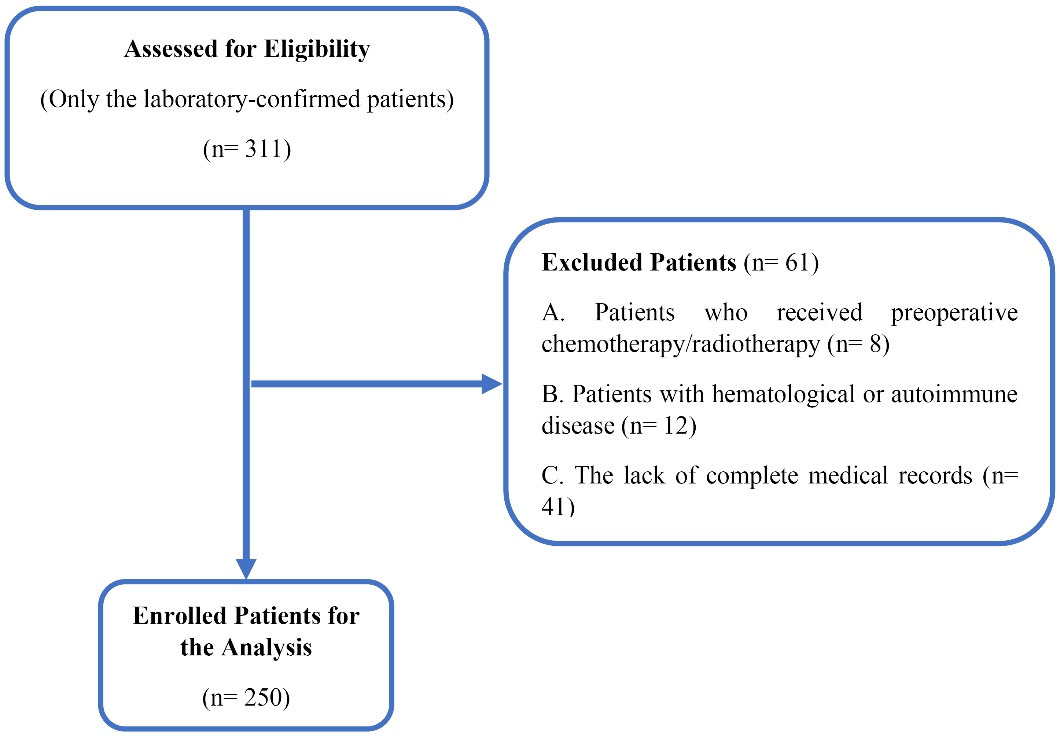
Figure 1.
The Flow Diagram for the Identification of Eligible Studies
.
The Flow Diagram for the Identification of Eligible Studies
The demographic, clinical, and laboratory data and outcomes of the patients were extracted from electronic case records. All laboratory findings of the patients upon admission were recorded. The primary parameters were selected to analyze if patients die during hospitalization or not, and if they need ICU stay or not. COVID-19 was diagnosed based on clinical presentations such as respiratory symptoms, fever, findings of viral pneumonia on computerized tomography, and/or positive COVID-19 polymerase chain reaction results based on the WHO guidance.19
All patients had definite outcomes, including death, discharge with ICU stay, and discharge without ICU. The current study was approved by the Medical Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1399.180).
Statistical Analysis
The normality of the data distribution was analyzed by the Kolmogorov-Smirnov test. The continuous values with normally distributed and skewed variables were reported as mean (standard deviation) and median (interquartile range), respectively. The categorical and nominal variables were reported as frequency (%). The comparison of the groups was made by the independent test: for continuous variables by the Mann-Whitney U test and for categorical variables by the chi-square test. Logistic regression was used to analyze the risk factors for ICU admission and death in COVID-19 patients in univariate and multivariate models. The covariate that was statistically significant in univariate analyses candidates for inclusion in multivariate models.
The area under the curve (AUC), also known as c-statistics, shows how much the model (predictive factor) is capable of differentiating between groups (e.g., survivor and non-survivor patients), and higher AUC reveals the better performance of the model in distinguishing between conditions.20 The receiver operating characteristic (ROC) curve as an effective method of evaluating the performance of diagnostic tests is defined as a plot of test sensitivity versus its specificity.21 AUC and ROC curve were used to determine the diagnostic value of studied parameters for death and ICU admission and also the sensitivity, specificity, and best cut-off values. A P value of less than 0.05 was considered significant. Then, SPSS version 25 was used for statistical analysis.
Results
Demographic Characteristics and Initial Laboratory Indices
A total of 250 patients were included, of which 58 (23.2%) patients died during hospitalization and were considered the non-survivor group, and the remaining 192 (76.8%) patients were regarded as the survivor group. Among the 250 consecutive patients with COVID-19 confirmed diagnosis, 127 (50.8%) cases were admitted to ICU at least for one day (the ICU admission group), and 123 were not admitted to ICU (the non-ICU admission group). The mean age of participants was 61.44 ± 16.47 years, and the mean age of survivors was significantly lower than that of non-survivors (P = 0.001). Further, the mean age of patients in the non-ICU admission group was significantly lower than that in the ICU admission group (P = 0.002). In terms of underlying diseases, ischemic heart disease (IHD) (P< 0.001), respiratory disease (P= 0.001), and hypertension (P= 0.05) were more frequently observed in ICU admission and non-survivor groups. Moreover, the frequency of hypertension was significantly higher in ICU-admitted patients compared with non-ICU admission cases (P= 0.03). In addition, the leucocytes, neutrophil, and lactate dehydrogenase (LDH) were significantly higher in ICU admission and non-survivor groups, whereas the level of hemoglobin and lymphocytes were significantly lower in ICU admission and non-survivor groups. The median level of NLR was 9.83 and 5.62 in the non-survivor and survivor groups, respectively (P< 0.001). Moreover, there were significant differences in derived neutrophil-to-lymphocyte ratio (dNLR) and PLR for non-survivors when compared to survivors. Data analysis demonstrated a significant difference in NLR, dNLR, and PLR that were higher in the ICU admission group compared to the non-ICU admission group. However, the platelet-to-white blood cell ratio (PWR) was significantly lower in ICU-admitted patients. Table 1 presents the demographic data and clinical laboratory data of hospitalized COVID-19 patients stratified by survival and ICU admission status.
Table 1.
Demographic and Clinical Laboratory Data of Hospitalized Patients with COVID-19 Stratified by Survival and ICU Admission Status
|
Variables
|
ICU Admission Status
|
Survival Status
|
|
Total (N=250)
|
ICU Admission (n=127)
|
Non-ICU Admission (n=123)
|
P
Value*
|
Survivors (n=192)
|
Non-survivors (n=58)
|
P
Value*
|
| Age (y), mean ± SD |
61.44 ± 16.47 |
64.64 ± 16.85 |
58.14 ± 15.46 |
0.002 |
59.30 ± 16.44 |
68.53 ± 14.68 |
0.001 |
| Gender n (%) |
0.96 |
|
|
0.46 |
| Male |
144 (57.6) |
73 (57.5) |
71 (57.7) |
113 (58.9) |
31 (53.4) |
| Female |
106 (42.4) |
54 (42.5) |
52 (42.3) |
79 (41.4) |
27 (46.6) |
| Comorbidities n (%) |
| Hypertension |
92 (36.8) |
55 (43.3) |
37 (30.1) |
0.03 |
65 (33.9) |
27 (46.6) |
0.07 |
| Diabetes |
53 (21.2) |
29 (22.8) |
24 (19.5) |
0.52 |
42 (21.9) |
11 (19.0) |
0.63 |
| IHD |
47 (18.8) |
39 (30.7) |
8 (6.5) |
< 0.001 |
26 (13.5) |
31 (36.2) |
< 0.001 |
| CKD |
25 (10) |
16 (12.6) |
9 (7.3) |
0.16 |
18 (9.4) |
7 (12.1) |
0.54 |
| Respiratory diseases |
26 (10.4) |
19 (15.0) |
7 (5.7)) |
0.01 |
13 (6.8) |
13 (22.4) |
0.001 |
| WBC median (IQR) |
8300
(5700, 11400) |
9400
(6700, 12600) |
7000
(5200, 10500) |
< 0.001 |
9600
(6775, 13450) |
8000
(5325, 11200) |
0.02 |
| HB mean (SD) |
13.10 (2.24) |
12.89 (2.39) |
13.46 (1.99) |
0.04** |
12.68 (2.20) |
13.28 (2.24) |
0.03** |
| PLT median (IQR) ˟ 103 |
196
(155, 246) |
205
(164, 248) |
188
(153, 245) |
0.12 |
207
(166 271) |
193
(155, 243) |
0.14 |
| PMN median (IQR) |
6691
(4429, 9591) |
7953
(5650, 10720) |
5380
(3860, 8307) |
< 0.001 |
8014
(5847, 11813) |
6391
(4117, 9211) |
0.002 |
| LYMPH median (IQR) |
995
(845, 1341) |
880
(703, 1086) |
1180
(980, 1489) |
< 0.001 |
791
(628., 934) |
1065
(898, 1426) |
< 0.001 |
| LDH median (IQR) |
607
(430, 598) |
684
(474.5, 1094) |
542
(414, 688) |
0.001 |
685
(482, 1103) |
575
(422, 830) |
0.03 |
| NLR median (IQR) |
6.62
(4.45, 9.48) |
2.77
(6.48, 11.16) |
4.70
(3.19, 6.60) |
< 0.001 |
9.83
(8.14, 14.16) |
5.62
(3.82, 8.22) |
< 0.001 |
| dNLR median (IQR) |
4.48
(3.34, 6.37) |
2.11
(4.29, 7.36) |
3.46
(2.41, 4.64) |
< 0.001 |
5.97
(4.91, 8.11) |
4.0
(2.93, 5.73) |
< 0.001 |
| PLR median (IQR) |
194.17
(148.41, 238.31) |
83.59
(189.13, 271.69) |
163.26
(133.28, 193.25) |
< 0.001 |
292.98
(224.58, 35.61) |
175.40
(138.28, 213.6) |
< 0.001 |
| PWR median (IQR) |
24.28
(17.90, 32.56) |
9.02
(16.86, 29.87) |
26.86
(20.73, 35.34) |
0.001 |
21.81
(16.79, 29.93) |
25.66
(18.14, 33.19) |
0.06 |
Note. COVID-19: Coronavirus disease 2019; ICU: Intensive care unit; SD: Standard deviation; IHD: Ischemic heart disease; HB: Hemoglobin; WBC: white blood cell; PLT: Platelet; IQR: Interquartile range; LYMPH: Lymphocyte; PMN: Polymorphonuclear; LDH: Lactate dehydrogenase; NLR: Neutrophil-to-lymphocyte ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio.
Univariate and Multivariate Logistic Regression Analysis to Predict Survival and ICU Admission of COVID-19 in Hospitalized Patients
Univariate logistic regression analysis indicated that all variables in the initial comparison to predict death from COVID-19 in hospitalized patients except for hemoglobin (Odds ratio [OR]: 0.89, P = 0.08) were statistically significant in univariate logistic regression analysis. Multivariate logistic regression analysis only revealed hypertension (OR: 3.18, P = 0.02) as an independent mortality risk factor in hospitalized COVID-19 patients (Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Analysis of Mortality Risk Factors of Hospitalized COVID-19 Patients
|
Variables
|
Univariate Model
|
Multivariate Model
|
|
OR
|
95% CI
|
P
Value
|
OR
|
95% CI
|
P
Value
|
| Age |
1.03 |
1.01-1.05 |
0.001 |
1.004 |
0.99-1.03 |
0.79 |
| Hypertension |
1.70 |
0.93-3.08 |
0.08 |
3.18 |
1.17, 8.64 |
0.02 |
| IHD |
3.62 |
1.14-7.12 |
< 0.001 |
2.60 |
0.84-7.99 |
0.09 |
| Respiratory diseases |
3.97 |
1.72-9.17 |
0.001 |
2.31 |
0.57-9.24 |
0.23 |
| WBC |
1.00 |
1.001-1.003 |
0.007 |
1.00 |
0.99-1.003 |
0.92 |
| HB |
0.89 |
0.77-1.01 |
0.08 |
0.96 |
0.77-1.18 |
0.71 |
| PLT |
1.00 |
1.00-1.00 |
0.006 |
1.00 |
1.00-1.00 |
0.71 |
| LYMPH |
0.99 |
0.99-0.99 |
< 0.001 |
0.99 |
0.98-1.005 |
0.40 |
| PMN |
1.00 |
1.00-1.00 |
< 0.001 |
1.00 |
0.99-1.003 |
0.86 |
| LDH |
1.00 |
1.00-1.00 |
0.05 |
1.00 |
1.00-1.00 |
0.64 |
| NLR |
1.50 |
1.33-1.70 |
< 0.001 |
1.44 |
0.61-3.36 |
0.39 |
| dNLR |
1.44 |
1.26-1.64 |
< 0.001 |
0.82 |
0.41-1.64 |
0.58 |
| PLR |
1.01 |
1.01-1.02 |
< 0.001 |
0.99 |
0.98-1.01 |
0.84 |
| PWR |
0.98 |
0.95-1.01 |
0.33 |
|
|
|
Note. COVID-19: Coronavirus disease 2019; OD: Odds ratio; CI: Confidence interval; IHD: Ischemic heart disease; WBC: White blood cell; HB: Hemoglobin; PLT: Platelet; LYMPH: Lymphocyte; PMN: Polymorphonuclear; LDH: Lactate dehydrogenase; NLR: Neutrophil-to-lymphocyte ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; IQR: Interquartile range.
Logistic regression analysis was also conducted for statistically different characteristics in the initial comparison to predict the ICU admission of hospitalized COVID-19 patients (Table 3).
Table 3.
Univariate and Multivariate Logistic Regression Analysis of Risk Factors for ICU Admission of Hospitalized COVID-19 Patients
|
Variables
|
Univariate Model
|
Multivariate Model
|
|
OR
|
95% CI
|
P
-value
|
OR
|
95% CI
|
P
-value
|
| Age |
1.02 |
1.00-1.04 |
0.002 |
0.99 |
0.96-1.02 |
0.58 |
| Hypertension |
1.77 |
1.06-2.99 |
0.03 |
4.00 |
1.69-9.48 |
0.002 |
| IHD |
6.39 |
2.83-14.31 |
< 0.001 |
5.15 |
1.53-17.32 |
0.008 |
| Respiratory diseases |
2.91 |
1.17-7.20 |
0.02 |
2.08 |
0.50-8.68 |
0.31 |
| WBC |
1.77 |
1.06-2.99 |
0.03 |
1.00 |
0.99-1.002 |
0.58 |
| HB |
6.39 |
2.83-14.31 |
< 0.001 |
0.88 |
0.73-1.05 |
0.16 |
| PLT |
2.91 |
1.17-7.20 |
0.02 |
1.00 |
1.000-1.000 |
0.96 |
| LYMPH |
1.77 |
1.06-2.99 |
0.03 |
1.00 |
0.99-1.003 |
0.94 |
| PMN |
6.39 |
2.83-14.31 |
< 0.001 |
0.99 |
0.99-1.001 |
0.57 |
| LDH |
2.91 |
1.17-7.20 |
0.02 |
1.00 |
1.00-1.00 |
0.19 |
| NLR |
1.63 |
1.43-1.86 |
< 0.001 |
1.06 |
0.53-2.14 |
0.85 |
| dNLR |
1.64 |
1.40-1.93 |
< 0.001 |
1.08 |
0.58-2.02 |
0.78 |
| PLR |
1.02 |
1.01-1.03 |
< 0.001 |
1.02 |
1.00-1.01 |
0.05 |
| PWR |
0.97 |
0.94-0.99 |
0.01 |
0.91 |
0.79-1.04 |
0.18 |
Note. COVID-19: Coronavirus disease 2019; OD: Odds ratio; CI: Confidence interval; IHD: Ischemic heart disease; WBC: White blood cell; HB: Hemoglobin; PLT: Platelet; LYMPH: Lymphocyte; PMN: Polymorphonuclear; LDH: Lactate dehydrogenase; NLR: Neutrophil-to-lymphocyte ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; ICU: Intensive care unit.
Univariate logistic regression analysis showed that all significant variables in the initial comparison were still significant. Furthermore, multivariate logistic regression analysis revealed that PLR (OR: 1.02, P = 0.05), hypertension (OR: 4.00, P = 0.002), and IHD (OR: 5.15, P = 0.008) were independent risk factors of ICU admission in hospitalized COVID-19 patients.
Calculated Cut-off Values of Laboratory Results to Predict Death Rate and ICU Admission of COVID-19 in Hospitalized Patients
We used ROC curve analysis to calculate optimal cut-off values of laboratory results. Figure 2 depicts the result of the ROC curve analysis of NLR, PLR, PWR, and dNLR for predicting death in hospitalized patients with COVID-19, and Table 4 shows the performance of studied indices for predicting mortality rate in patients with COVID-19.
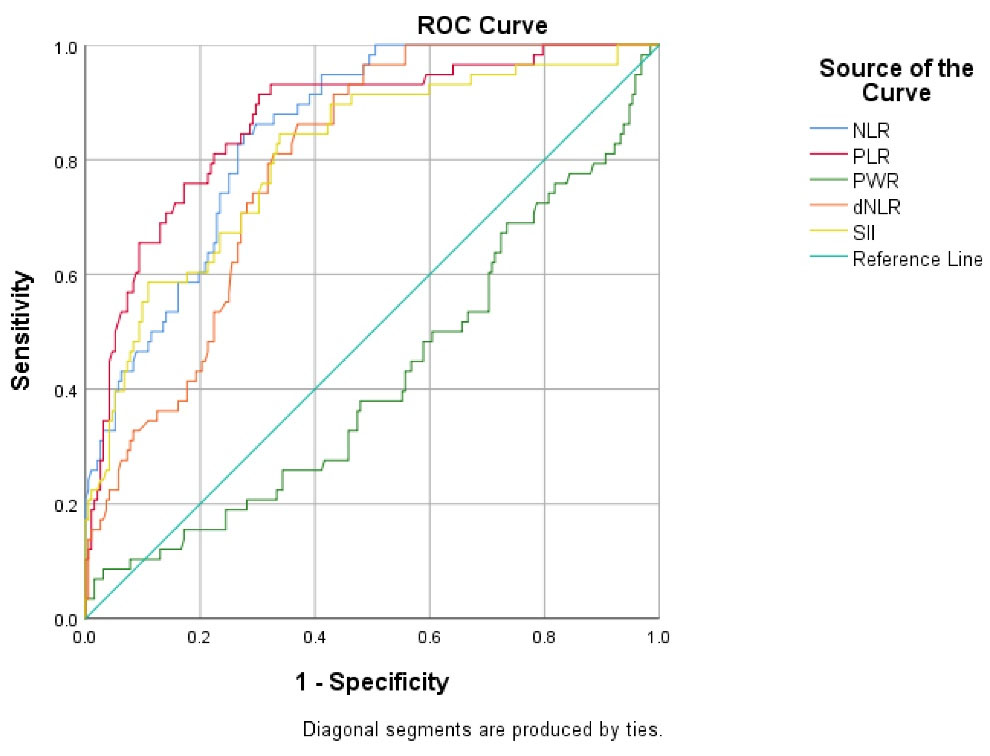
Figure 2.
The ROC Curve Analysis of NLR, PLR, PWR, and dNLR for Predicting Death in Hospitalized Patients with COVID-19. Note. ROC: Receiver operating characteristic; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; COVID-19: Coronavirus disease 2019
.
The ROC Curve Analysis of NLR, PLR, PWR, and dNLR for Predicting Death in Hospitalized Patients with COVID-19. Note. ROC: Receiver operating characteristic; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; COVID-19: Coronavirus disease 2019
Table 4.
The Performance of Studied Indices for Predicting Death in Patients with COVID-19
|
Variables
|
Cut-off
|
AUC
|
95% CI
|
P
Value
|
Sensitivity (%)
|
Specificity (%)
|
| NLR |
7.61 |
0.847 |
0.79, 0.89 |
< 0.001 |
84 |
72 |
| dNLR |
4.54 |
0.790 |
0.73, 0.84 |
< 0.001 |
86 |
63 |
| PLR |
202.7 |
0.866 |
0.81, 0.91 |
< 0.001 |
91 |
69.8 |
| PWR |
45.6 |
0.419 |
0.33, 0.50 |
0.06 |
8 |
96 |
Note. COVID-19: Coronavirus disease 2019; AUC: Areas under the curve; CI: Confidence interval; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio.
AUC of NLR (0.847, 95% confidence interval [CI]: 0.79-0.89, P< 0.001), PLR (0.866, 95% CI: 0.81-0.91, P< 0.001), and dNLR (0.790, 95% CI: 0.73-0.84, P< 0.001) was significant for predicting the death of patients with COVID-19. NLR level of 7.61, PLR level of 202.7, and dNLR level of 4.54 could significantly predict death in patients with COVID-19 as optimal cut-off values. Moreover, the highest sensitivity was related to PLR (91%), and the highest specificity was related to NLR (72%).
Based on ROC curve analysis (Figure 3 and Table 5), AUC of NLR (0.808, 95% CI: 0.75-0.86, P< 0.001), PLR (0.771, 95% CI: 0.71-0.83, P< 0.001), and dNLR (0.773, 95% CI: 0.71-0.83, P < 0.001) was significant for predicting the ICU admission of patients with COVID-19.
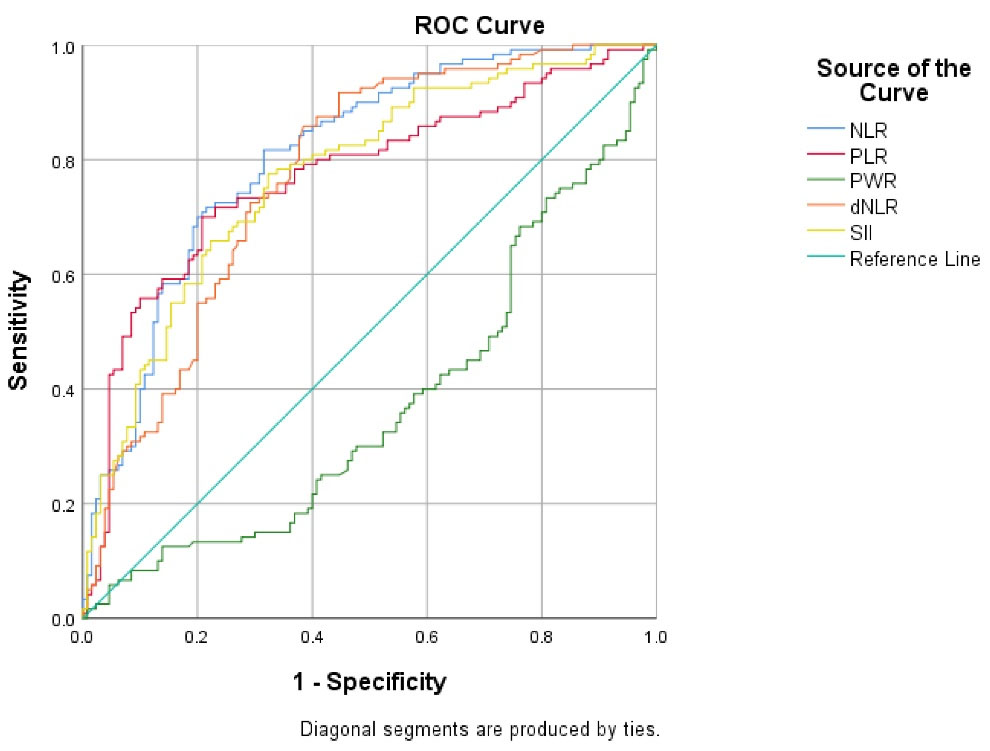
Figure 3.
The ROC Curve Analysis of NLR, PLR, PWR, and dNLR for Predicting ICU Admission in Hospitalized Patients with COVID-19. Note. ROC: Receiver operating characteristic; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; ICU: Intensive care unit; COVID-19: Coronavirus disease 2019
.
The ROC Curve Analysis of NLR, PLR, PWR, and dNLR for Predicting ICU Admission in Hospitalized Patients with COVID-19. Note. ROC: Receiver operating characteristic; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; ICU: Intensive care unit; COVID-19: Coronavirus disease 2019
Table 5.
The Performance of Studied Indices for Predicting ICU Admission in Patients with COVID-19
|
Variables
|
Cut-off
|
AUC
|
95% CI
|
P
-value
|
Sensitivity (%)
|
Specificity (%)
|
| NLR |
6.22 |
0.808 |
0.75, 0.86 |
< 0.001 |
84 |
72 |
| dNLR |
3.98 |
0.773 |
0.71, 0.83 |
< 0.001 |
85 |
61 |
| PLR |
202.78 |
0.771 |
0.71, 0.83 |
< 0.001 |
70 |
79 |
| PWR |
43.92 |
0.373 |
0.30, 0.44 |
0.001 |
5 |
95 |
Note. ICU: Intensive care unit; COVID-19: Coronavirus disease 2019; AUC: Areas under the curve; CI: Confidence interval; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; dNLR: Derived neutrophil-to-lymphocyte ratio; PWR: Platelet-to-white blood cell ratio.
In addition, ROC curve analysis of PWR (0.373, 95% CI: 0.30-0.44, P= 0.001) showed that the level of PWR can be used as a predicting factor for ICU admission of patients with COVID-19, and the lower level of PWR is related to the higher probability of ICU admission. Moreover, the optimal cut-off values for NLR, PLR, and dNLR were 6.22, 202.7, and 43.98, respectively, which could significantly predict the ICU admission of patients with COVID-19. Furthermore, the highest sensitivity was related to NLR (81%), and the highest specificity was related to PLR (79%).
Discussion
COVID-19 has elicited a rapid spread of outbreak with human-to-human transmission.22,23 COVID-19 pneumonia oat the early stage was not extremely severe. At the beginning of the severe stage, patients showed severe pneumonia and passed away on 7-14 days. Given that several controversial declarations about predictive indices for COVID-19 severe illness have been published so far, a necessary need for the prediction of the progression chance of COVID-19 is felt, and more investigations are pivotal in this regard. The analysis of demographic data in our study revealed that the age of surviving from COVID-19 was significantly lower compared to non-survivors. Similar to our results, several reports from previous investigations revealed that disease severity was significantly related to age.24-26 Romero Starke et al27 conducted a systematic review and meta-analysis and found that the risk of in-hospital and case mortality increases per age by 5.7% and 7.4%, respectively, while the risk of hospitalization increases by 3.4% per age. However, there was no significant increased risk for ICU admission by age. Likewise, Grasselli et al28 reported no age differences between COVID-19 patients admitted to ICU compared to the general positive population.
Recently, it has been documented that the NLR level, as a novel inflammatory index, is an early risk factor for the prediction of ICU admission rate and the severity of CODID-19 disease.29,30 As hypothesized in recent studies, patients with age ≥ 50 and NLR ≥ 3.13 are considered severe cases, and they should be bedridden in ICUs.31 Furthermore, another study revealed that the decrease in lymphocyte count indicates the disease progress.32 A large body of scientific literature has declared that blood lymphocyte and neutrophil-related indices may be a potential predictor of this disease. For more validation of this declaration, the present study evaluated these indices in the Iranian population with COVID-19. In the recent decay, the NLR index was considered a marker of the severity of bacterial infections.33
In the current study, the data of 250 COVID-19 patients were analyzed, and the laboratory indices were presented. The results indicated that the NLR factor can be one of the most significant factors in predicting severe disease. In support of our finding, Liu et al31 compared NLR with CURB-65 and MuLBSTA scoring systems. They documented that NLR has higher AUC, sensitivity, and specificity than the other two models. Recently, Yang et al29 have reported that increased NLR is an independent prognostic factor that may affect disease progression in COVID-19 patients. Our results were consistent with previously published studies regarding the relationship between NLR and COVID-19 progression. Based on these findings, it can be hypothesized that the immune response initiated by viral infection mostly depends on lymphocyte,34 whereas systematic inflammation drastically suppresses cellular immunity, significantly decreasing CD4 + T lymphocytes and increasing CD8 + T lymphocyte.35 Another possible mechanism for the reduction of lymphocytes in COVID-19 infection is that lymphocytes are the target of the virus because the angiotensin-converting enzyme 2 receptor of the virus is expressed on lymphocytes, too.36,37 On the other hand, neutrophilia may stem from the cytokine storm initiated by virus infection.38,39 Thus, virus-related inflammation escalates the NLR factor.
In this study, the optimal cut-off values for NLR, PLR, dNLR, and PWR were obtained via the ROC curve. The optimal threshold of 7.61 for NLR showed a prognostic possibility of the mortality rate of disease. The highest level of AUC for predicting death in COVID-19 patients was related to PLR (0.866) with a sensitivity of 91% and specificity of 69%. Moreover, our analysis publicized that along with NLR, PLR, and dNLR may also be used as a predictive value for identifying subjects needing ICU admission. Recent investigations displayed that the authentication of PLR is required.40 In the present study, the optimal threshold value of PLR was identified with the highest sensitivity (91%) with an acceptable specificity (69.8%). The AUC of NLR achieved the highest value (0.808) at the optimal cut-off point to predict ICU admission (sensitivity and specificity of NLR is 84% and 72%, respectively). Our outcomes are similar to previous studies, and a higher NLR value signifies a predictive factor for the severity of COVID-19. Along with the NLR factor, we observed the optimum AUC, sensitivity, and specificity of the threshold value of dNLR (0.773, 85%, and 61%, respectively) and PLR (0.771, 70%, and 79%, respectively). According to our results, not only NLR but also dNLR and PLR could have prognostic value for predicting ICU admission in COVID-19 patients. The useful application of NLR and PLR has been documented in various diseases, including autoimmune disease,41 tumors,42,43 bacterial pneumonia,44 and secondary pulmonary infectious diseases.45 It has been well-documented that these indices could be able to predict survival rate and the severity of the above-mentioned diseases. However, the application of dNLR and PWR in COVID-19 pneumonia was poorly reported. Based on our study and recently published documents, it can be said that NLR, PLR, Dnlr, and PWR indicate the progression trend of COVID-19 and can be used as a prognostic value for COVID-19 conditions.
The findings of the current study revealed that increased NLR was an independent prognostic factor for patients with COVID-19. Further, according to obtained values of AUC, sensitivity, and specificity for dNLR, it could be concluded that dNLR can be applicable for predicting death and ICU bed management in COVID-19 patients. Another finding of the current study is related to the PWR index. In line with other scientific reports, our obtained data showed that PWR has a reverse relation with ICU admission and mortality rate from COVID-19. Due to the huge medical and economic burden of COVID-19, NLR and other novel parameters are simple, fast, and economical to achieve from blood samples compared to other factors such as interleukin-6, which can assist clinicians recognize the severely ill patients with COVID-19.46 Although the prognostic value of NLR, PLR, and dNLR factors has been documented recently in COVID-19 patients, the present study further approved these prognostic factors in the Iranian COVID-19 population.
The current study has a number of limitations. First, it is a retrospective study in the northwest of Iran, and the results have low external validation; therefore, more studies in other centers are required to achieve more acceptable results. Second, different physicians contributed to the process of patients’ management during hospitalization which may affect the outcome of COVID-19 patients due to different treatment approaches. Therefore, it seems that the implementation of prospective cohort studies involving more patients would allow more reliable clinical conclusions.
Conclusions
In conclusion, this retrospective cohort study declares that age and the NLR, PLR, PWR, and dNLR are valuable factors for predicting ICU admission and mortality rate of patients with COVID-19. Due to the restriction of hospital facilities, the early identification of patients with a higher risk for progression and further need for ICU admission is important. Monitoring of COVID-19 severity predictors could help clinicians to recognize and follow up these patients. Further research is required to authorize our findings in other cohort studies and to associate the predictive potential of NLR and other novel parameters.
Acknowledgments
We would like to thank all staff of the archive office for their kind full cooperation and all nurses and staff of the ward at Imam Reza hospital for their kind full cares of all patients.
Funding
The research protocol was approved and supported by the Student Research Committee of Tabriz University of Medical Sciences (Grant number: 65263).
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethical approval
Written informed consent was obtained from each patient during questionnaire administration for the collection and analysis of applicable clinical data. Further, the protocol was approved by the Regional Ethics Committee headed by the Vice-chancellor of Research and Development at Tabriz University of Medical Sciences (Ethical approval code: IR.TBZMED.REC.1399.180).
Consent for publication
Not applicable.
Conflict of interests
The authors declare no competing interests.
References
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061-9. doi: 10.1001/jama.2020.1585 [Crossref] [ Google Scholar]
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17(5):259-60. doi: 10.1038/s41569-020-0360-5 [Crossref] [ Google Scholar]
- Molaei H, Khedmat L, Nemati E, Rostami Z, Saadat SH. Iranian kidney transplant recipients with COVID-19 infection: clinical outcomes and cytomegalovirus coinfection. Transpl Infect Dis 2021; 23(1):e13455. doi: 10.1111/tid.13455 [Crossref] [ Google Scholar]
- Hashemifesharaki R, Gharibzahedi SMT. Future nutrient-dense diets rich in vitamin D: a new insight toward the reduction of adverse impacts of viral infections similar to COVID-19. Nutrire 2020; 45(2):19. doi: 10.1186/s41110-020-00122-4 [Crossref] [ Google Scholar]
- Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med 2020; 8(5):433-4. doi: 10.1016/s2213-2600(20)30127-2 [Crossref] [ Google Scholar]
- Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 2020; 24(1):108. doi: 10.1186/s13054-020-2833-7 [Crossref] [ Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229):1054-62. doi: 10.1016/s0140-6736(20)30566-3 [Crossref] [ Google Scholar]
- Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 2020; 80(6):656-65. doi: 10.1016/j.jinf.2020.03.041 [Crossref] [ Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382(8):727-33. doi: 10.1056/NEJMoa2001017 [Crossref] [ Google Scholar]
- Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015; 12(10):584-96. doi: 10.1038/nrclinonc.2015.105 [Crossref] [ Google Scholar]
- Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev 2016; 273(1):329-43. doi: 10.1111/imr.12459 [Crossref] [ Google Scholar]
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126(5):582-8. doi: 10.1182/blood-2014-08-531582 [Crossref] [ Google Scholar]
- Meng X, Wei G, Chang Q, Peng R, Shi G, Zheng P. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int J Infect Dis 2016; 45:72-7. doi: 10.1016/j.ijid.2016.02.025 [Crossref] [ Google Scholar]
- Curbelo J, Luquero Bueno S, Galván-Román JM, Ortega-Gómez M, Rajas O, Fernández-Jiménez G. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: Importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. PLoS One 2017; 12(3):e0173947. doi: 10.1371/journal.pone.0173947 [Crossref] [ Google Scholar]
- Huang Y, Liu A, Liang L, Jiang J, Luo H, Deng W. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol 2018; 64:10-5. doi: 10.1016/j.intimp.2018.08.022 [Crossref] [ Google Scholar]
- Zhao C, Wei Y, Chen D, Jin J, Chen H. Prognostic value of an inflammatory biomarker-based clinical algorithm in septic patients in the emergency department: an observational study. Int Immunopharmacol 2020; 80:106145. doi: 10.1016/j.intimp.2019.106145 [Crossref] [ Google Scholar]
- Fu H, Qin B, Hu Z, Ma N, Yang M, Wei T. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin Lab 2015; 61(3-4):269-73. doi: 10.7754/clin.lab.2014.140927 [Crossref] [ Google Scholar]
- Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol 2020; 92(10):1733-4. doi: 10.1002/jmv.25819 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease is Suspected: Interim Guidance, 13 March 2020. WHO; 2020.
- Muschelli J. ROC and AUC with a binary predictor: a potentially misleading metric. J Classif 2020; 37(3):696-708. doi: 10.1007/s00357-019-09345-1 [Crossref] [ Google Scholar]
- Park SH, Goo JM, Jo CH. Receiver operating characteristic (ROC) curve: practical review for radiologists. Korean J Radiol 2004; 5(1):11-8. doi: 10.3348/kjr.2004.5.1.11 [Crossref] [ Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 2020; 24:91-8. doi: 10.1016/j.jare.2020.03.005 [Crossref] [ Google Scholar]
- Hassanpour M, Rezaie J, Nouri M, Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect Genet Evol 2020; 85:104422. doi: 10.1016/j.meegid.2020.104422 [Crossref] [ Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229):1054-62. doi: 10.1016/s0140-6736(20)30566-3 [Crossref] [ Google Scholar]
- Chen L, Yu J, He W, Chen L, Yuan G, Dong F. Risk factors for death in 1859 subjects with COVID-19. Leukemia 2020; 34(8):2173-83. doi: 10.1038/s41375-020-0911-0 [Crossref] [ Google Scholar]
- Fois AG, Paliogiannis P, Scano V, Cau S, Babudieri S, Perra R. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules 2020; 25(23):5725. doi: 10.3390/molecules25235725 [Crossref] [ Google Scholar]
- Romero Starke K, Reissig D, Petereit-Haack G, Schmauder S, Nienhaus A, Seidler A. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health 2021; 6(12):e006434. doi: 10.1136/bmjgh-2021-006434 [Crossref] [ Google Scholar]
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323(16):1574-81. doi: 10.1001/jama.2020.5394 [Crossref] [ Google Scholar]
- Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020; 84:106504. doi: 10.1016/j.intimp.2020.106504 [Crossref] [ Google Scholar]
- Kerboua KE. NLR: a cost-effective nomogram to guide therapeutic interventions in COVID-19. Immunol Invest 2021; 50(1):92-100. doi: 10.1080/08820139.2020.1773850 [Crossref] [ Google Scholar]
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv [Preprint]. February 12, 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.02.10.20021584v1.
- Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis 2020; 71(6):1393-9. doi: 10.1093/cid/ciaa414 [Crossref] [ Google Scholar]
- Furutate R, Ishii T, Motegi T, Hattori K, Kusunoki Y, Gemma A. The neutrophil to lymphocyte ratio is related to disease severity and exacerbation in patients with chronic obstructive pulmonary disease. Intern Med 2016; 55(3):223-9. doi: 10.2169/internalmedicine.55.5772 [Crossref] [ Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71(15):762-8. doi: 10.1093/cid/ciaa248 [Crossref] [ Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4 + T lymphocytes. J Immunol 2007; 178(2):732-9. doi: 10.4049/jimmunol.178.2.732 [Crossref] [ Google Scholar]
- Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin- converting enzyme 2 as a potential drug target - a perspective. Endocr Metab Immune Disord Drug Targets 2020; 20(6):807-11. doi: 10.2174/1871530320666200427112902 [Crossref] [ Google Scholar]
- Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 2020;182(1):50-8.e8. 10.1016/j.cell.2020.05.027.
- Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 2021; 93(1):250-6. doi: 10.1002/jmv.26232 [Crossref] [ Google Scholar]
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39(5):529-39. doi: 10.1007/s00281-017-0629-x [Crossref] [ Google Scholar]
- Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B. Can we predict the severity of coronavirus disease 2019 with a routine blood test?. Pol Arch Intern Med 2020; 130(5):400-6. doi: 10.20452/pamw.15331 [Crossref] [ Google Scholar]
- Zeng Z, Wang C, Wang B, Wang N, Yang Y, Guo S. Prediction of neutrophil-to-lymphocyte ratio in the diagnosis and progression of autoimmune encephalitis. Neurosci Lett 2019; 694:129-35. doi: 10.1016/j.neulet.2018.12.003 [Crossref] [ Google Scholar]
- Zhou B, Deng J, Chen L, Zheng S. Preoperative neutrophil-to-lymphocyte ratio and tumor-related factors to predict lymph node metastasis in nonfunctioning pancreatic neuroendocrine tumors. Sci Rep 2017; 7(1):17506. doi: 10.1038/s41598-017-17885-y [Crossref] [ Google Scholar]
- Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol 2017; 115(4):470-9. doi: 10.1002/jso.24523 [Crossref] [ Google Scholar]
- Lee JH, Song S, Yoon SY, Lim CS, Song JW, Kim HS. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic markers for pneumonia severity. Br J Biomed Sci 2016; 73(3):140-2. doi: 10.1080/09674845.2016.1209898 [Crossref] [ Google Scholar]
- Karataş MB, İpek G, Onuk T, Güngör B, Durmuş G, Çanga Y. Assessment of prognostic value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with pulmonary embolism. Acta Cardiol Sin 2016; 32(3):313-20. doi: 10.6515/acs20151013a [Crossref] [ Google Scholar]
- Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020; 81(1):e6-e12. doi: 10.1016/j.jinf.2020.04.002 [Crossref] [ Google Scholar]