Int J Aging. 2024;2:e19.
doi: 10.34172/ija.2024.e19
Review Article
Obesity: Global Epidemiology, Trends, Risk Factors, and Clinical Aspects
Saeid Safiri 1, 2, 3, *  , Amin Daei Sorkhabi 4, Reza Aletaha 3, Sana Hamidi 1, Kimia Motlagh Asghari 1, Aila Sarkesh 1, Sina Janbaz Alamdary 1, Amir Ghaffari Jolfayi 5, Seyed Aria Nejadghaderi 6, Asra Fazlollahi 1, Reza Mohammadinasab 7, Mark J. M. Sullman 8, 9, Nahid Karamzad 10, 11, Fikrettin Sahin 12, Ali-Asghar Kolahi 13, *
, Amin Daei Sorkhabi 4, Reza Aletaha 3, Sana Hamidi 1, Kimia Motlagh Asghari 1, Aila Sarkesh 1, Sina Janbaz Alamdary 1, Amir Ghaffari Jolfayi 5, Seyed Aria Nejadghaderi 6, Asra Fazlollahi 1, Reza Mohammadinasab 7, Mark J. M. Sullman 8, 9, Nahid Karamzad 10, 11, Fikrettin Sahin 12, Ali-Asghar Kolahi 13, * 
Author information:
1Neurosciences Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
2Clinical Research Development Unit, Imam Reza General Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
3Social Determinants of Health Research Center, Department of Community Medicine, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
4Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
5Rajaie Cardiovascular Medical and Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
6HIV/STI Surveillance Research Center, and WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health Kerman University of Medical Sciences Kerman Iran
7Department of History of Medicine, School of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
8Department of Life and Health Sciences, University of Nicosia, Nicosia, Cyprus
9Department of Social Sciences, University of Nicosia, Nicosia, Cyprus
10Department of Persian Medicine, School of Traditional Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
11Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
12Department of Genetics and Bioengineering, Faculty of Engineering, Yeditepe University, Istanbul, Turkey
13Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Objectives:
To review the current literature on obesity risk factors, epidemiology, and trends, providing insights for effective prevention and intervention strategies.
Design:
Review article.
Setting(s):
Global.
Outcome measures:
A systematic search was performed using MEDLINE (via PubMed), Scopus, Web of Science, and Google Scholar up to January 2024. Studies on obesity’s history, epidemiology, risk factors, health impacts, and preventive or therapeutic approaches were included. Both primary and secondary studies were considered, excluding those in languages other than English, in vitro studies, and animal studies. No restrictions were applied regarding publication date or article type.
Results:
The overweight- and obesity-attributable burden of diseases has significantly increased, particularly among adults aged 60 and older, with the most severe effects observed in women aged≥75, highlighting a growing public health challenge and a markedly greater rate of increase in older adults compared to those under 60. The causes of obesity were found to be multifaceted, predominantly influenced by behavioral and environmental factors, with an imbalance between calorie intake and expenditure being the primary issue. The adverse health consequences of obesity have been well documented, with associations noted in various non-communicable diseases, including diabetes, cardiovascular diseases (CVDs), and musculoskeletal disorders.
Conclusions:
Understanding obesity-comorbidity links is vital to identifying high-risk individuals and prioritizing interventions. Limited access to effective weight management treatments remains a key barrier to improving health outcomes for those affected by obesity.
Keywords: Obesity, Overweight, Epidemiology, Risk factor, Worldwide, Disability
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The present study was supported by the Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 43005963).
Please cite this article as follows: Safiri S, Daei Sorkhabi A, Aletaha R, Hamidi S, Motlagh Asghari K, Sarkesh A, et al. Obesity: global epidemiology, trends, risk factors, and clinical aspects. Int J Aging. 2024;2: e19. doi: 10.34172/ija.2024.e19
Introduction
Obesity is a significant global public health concern.1 The World Health Organization (WHO) defines obesity as an abnormal or excessive accumulation of fat that poses a health risk, categorizing it as a body mass index (BMI) exceeding 30.2 In recent decades, the worldwide prevalence of obesity has escalated to pandemic levels.3-6 As reported by the WHO, the prevalence of obesity has more than tripled since 1975, with over 1 billion individuals classified as obese globally in 2020.2
Obesity is a multifaceted disease influenced by various factors and is regarded as a chronic, progressive condition rather than merely a risk factor for other ailments.7-9 The primary cause of this disease is a sustained energy imbalance between calorie intake and expenditure.10,11 Excess energy is converted into triglycerides and stored in adipose tissue depots, which enlarge, increasing body fat and weight gain.8 Several factors contribute to the onset of obesity, including genetic predisposition, neuroendocrine influences, environmental factors, sociocultural aspects, and lifestyle choices.12-17
Obesity poses a considerable health challenge, as it heightens the risk of metabolic disorders, including type 2 diabetes mellitus (T2DM) and fatty liver disease, as well as cardiovascular diseases (CVDs) such as myocardial infarction,18 hypertension (HTN), and stroke.19,20 Obesity is also linked to osteoarthritis, Alzheimer’s disease (AD), obstructive sleep apnea (OSA), depression, and some types of cancer.11,21-22 Furthermore, obesity and its associated comorbidities not only significantly impact the quality of life and overall well-being,23 but also place a staggering burden on healthcare systems.24
The above-mentioned findings, which highlight the rising prevalence of obesity and its association with various health conditions, underscore the strong need for a comprehensive understanding of obesity epidemiology to address this growing public health crisis. The present article aims to explore the diverse range of factors contributing to the development and progression of obesity. Genetic predisposition, environmental influences, lifestyle behaviors, and socio-cultural determinants all play significant roles in this complex issue. Understanding the risk factors associated with obesity is of paramount importance for designing effective prevention and intervention strategies. Additionally, this article provides a comprehensive overview of obesity, including its epidemiology, trends, and risk factors. Examining these aspects can provide us with deeper insights into the magnitude of the obesity problem, enabling the identification of high-risk populations and the subsequent development of more effective evidence-based interventions and policies.
Methods
The MEDLINE (via PubMed), Scopus, and Web of Science databases, along with Google Scholar, were systematically searched up to January 2024 to identify eligible studies using a combination of keywords pertaining to obesity, encompassing its history, epidemiology, risk factors, health consequences, and preventive and therapeutic approaches. No search filters, such as publication date or article type, were applied in this regard. The review incorporated both primary and secondary studies that examined the relationship between biological or psychological factors and obesity. Additionally, our analysis included research presenting data on the epidemiological attributes or burden of obesity at global, regional, and national levels, as well as studies assessing the relationship between various disorders and obesity in humans. Studies conducted in languages other than English, along with in vitro and animal studies, were excluded from the review.
A Brief History of Obesity
Humanity has grappled with obesity since ancient times, as evidenced by excavations from the Paleolithic era.25 One of the oldest surviving depictions of a person with excessive body weight dates back to over 2000-3000 years old.25 The Venus of Willendorf, a famous statue portraying a woman with prominently delineated breasts, abdomen, and thighs, was unearthed in 1908.26 The historical imagery or metaphor of excess body fat is intriguing, considering the prevalent food shortages, the constant need to hunt animals, and the struggle for survival.25 The stone tombs of several high-ranking officials from ancient Egypt suggest that obesity was not uncommon among them.25 Additionally, large human figurines have been discovered during archaeological excavations in ancient Mesopotamia, Aztec, and Inka cultures.25
As society and science progressed, overweight became recognized as a medical concern.27,28 The writings of Hippocrates contain the earliest explicit mention of obesity as a significant clinical issue.27 According to Hippocrates, obesity was attributed to an overall surplus of the four humors (fluids) that constitute a healthy human, including blood, black bile, yellow bile, and phlegm.27 Hippocrates was the first medical professional to assert that obesity increases the risk of early death, CVDs, menstrual problems in women, and infertility. He also advocated for dietary restrictions and exercise as effective means to reduce excess body weight.27,28 Soranus of Ephesus, another Greek physician, asserted that exercising, following a diet with extremely few calories, and intensifying diuresis could all aid in weight loss. Two Roman physicians, Galen and Areteus, also espoused similar views in this regard.29 Galen described a condition he named “polysarkia” (poly = numerous and sark = flesh), which is now recognized as morbid obesity.30,31 He observed that individuals with this condition faced difficulties in cleaning themselves, breathing easily, giving birth, or walking without perspiration.30,31
The “dark ages” in Europe witnessed few notable medical advances until the Renaissance.32 Meanwhile, medical research and other scientific fields were thriving in the Middle East.32 Influenced by historical figures such as Hippocrates and Galen, Muhammad ibn Zakariya Al-Razi discussed obesity in his book Al-Hawi Fit-Tibb (an encyclopedia of medicine).33,34 Other Persian doctors, such as Ibn Sina (Avicenna), Ibn Hubal Al–Baghdad, and Ibn Al–Nafis, focused their research on the pathophysiology of obesity and its associated complications.34 They explored the connection between obesity and respiratory, endocrine, and cardiovascular conditions, as well as reduced fertility.34
In the Baroque era, being overweight began to symbolize health and happiness again, largely due to the resurgence of plagues, poverty, and starvation.35 This sentiment is reflected in the works of writers, paintings, and even the descriptions of fictional characters such as Sir John Falstaff, who appeared in three plays by William Shakespeare.35,36 English physician Thomas Short produced the first monograph on obesity in 1727.37 Short advocated for a successful weight-loss strategy involving the elimination of factors that contribute to obesity, such as a sedentary lifestyle and unrestrained overeating, in order to restore the body’s natural equilibrium.37,38 Adolphe Quetelet’s cross-sectional research in 1835 concluded that measuring weight adjusted for height would be the most effective means to identify obesity.39,40 Ancel Keys coined the term “body mass index (BMI)” in 1972, originally known as the “Quetelet index”, which is still used in clinical settings today.39,40
Hassall provided the first descriptions of adipocyte growth and evolution in 1849,41 and proposed that certain kinds of obesity, now known as hyperplastic obesity, are caused by an increase in fat cells.41 In 1967, Stewart first used behavioral therapy to treat obesity.42 Despite advances in dietetics, the increasing popularity of physical exercise, and attempts at pharmaceutical therapies, efforts to combat the obesity epidemic have been ineffective.42 Currently, surgery is the most common approach for treating morbid obesity.42 Initially, procedures that removed a portion of the small intestine to reduce the absorption area were dominant, but they had several drawbacks.42 Methods to reduce stomach capacity have been employed since the 1970s.42 Mason and Ito implemented the first such procedure, the Roux-en-Y gastric bypass (RYGB).42 Better surgical procedures were gradually developed, leading to the introduction of gastric banding in the 1980s.43 The introduction of the less invasive laparoscopic technique in the last decade of the 20th century greatly increased the popularity of bariatric surgery.43,44 In 1986, an international association was founded for the study of obesity.25
The perception of obesity as a symbol of affluence and wealth has changed significantly over time, and obesity has now emerged as one of the most significant health challenges facing society in the twenty-first century.45 Despite the remarkable progress made in identifying the underlying causes, triggers, and effects of obesity over the last 25–30 years, combating a clinical issue that has existed for thousands of years remains one of the most serious problems of modern medicine.45
Global Epidemiology
In 2019, the summary exposure value of high BMI ranged from 56.5 (43.4–66.2) in Qatar to 4.52 (2.27–8.08) in the Democratic People’s Republic of Korea per 100,000 population. The United Arab Emirates had the second highest exposure value, with 53.6 (41.7–61.8), while Somalia had the second lowest value of 4.5 (2.5–7.4), the details of which are shown in Figure 1.46
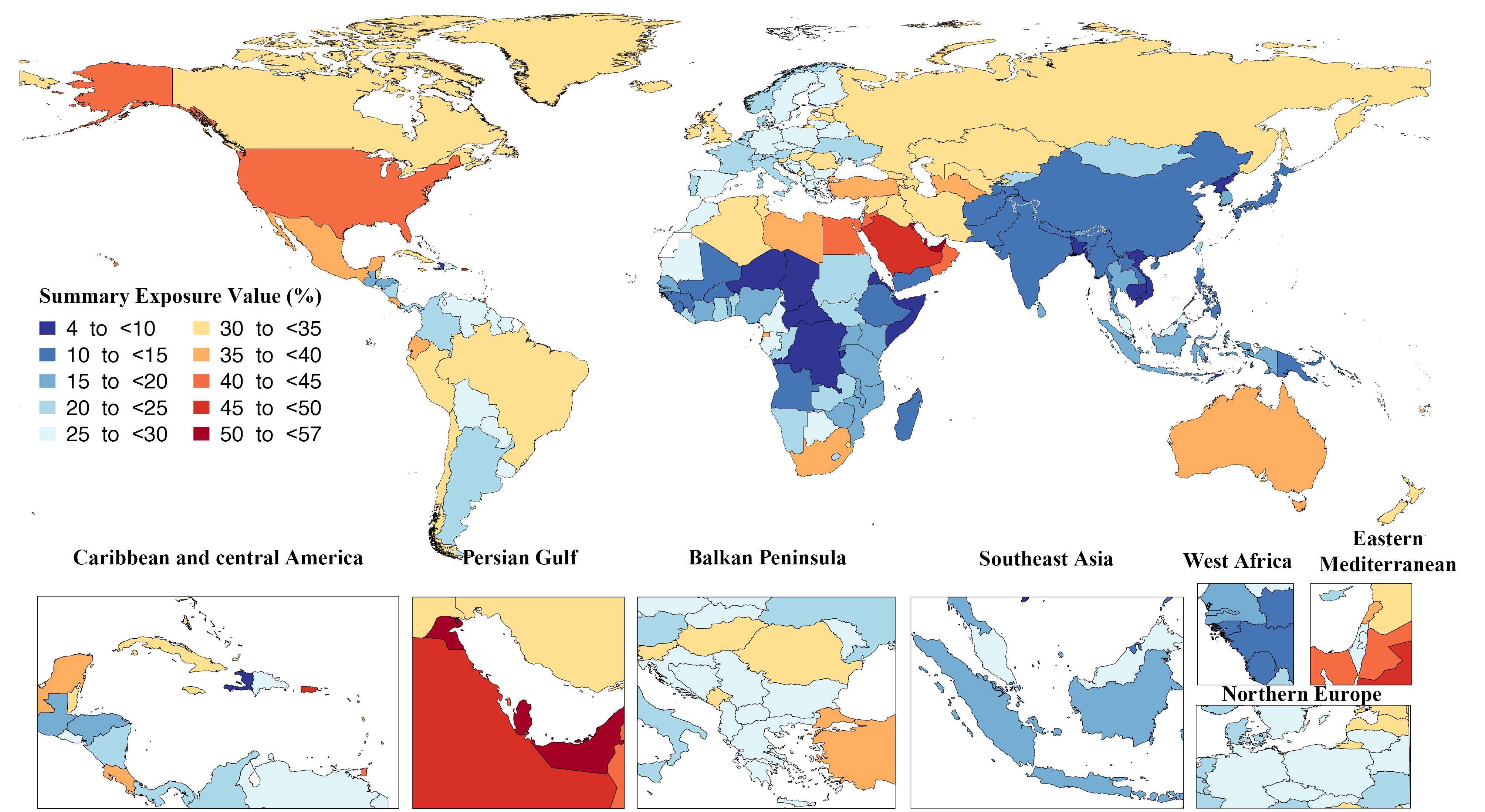
Figure 1.
Summary Exposure Value of the Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available on http://ghdx.healthdata.org/gbd-results-tool
.
Summary Exposure Value of the Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available on http://ghdx.healthdata.org/gbd-results-tool
Fiji and the Cook Islands had the highest percentage of disability-adjusted life years (DALYs) due to high BMI in all ages, with 24.5 (18.5–29.5) and 24.2 (17.9–29.60), respectively. Conversely, Somalia, Chad, and Niger had the lowest percentage of disability with 0.6 (0.2–1.2), 0.8 (0.4–1.3), and 0.8 (0.5–1.3), respectively, in 2019 per 100 000 population (Figure 2).46 In 2019, the age-standardized DALY rates due to high BMI varied from 10000.5 (6266.5–14159.1) in Kiribati to 503.2 (199.9–888.7) in Japan per 100 000 population (Figure 3).46
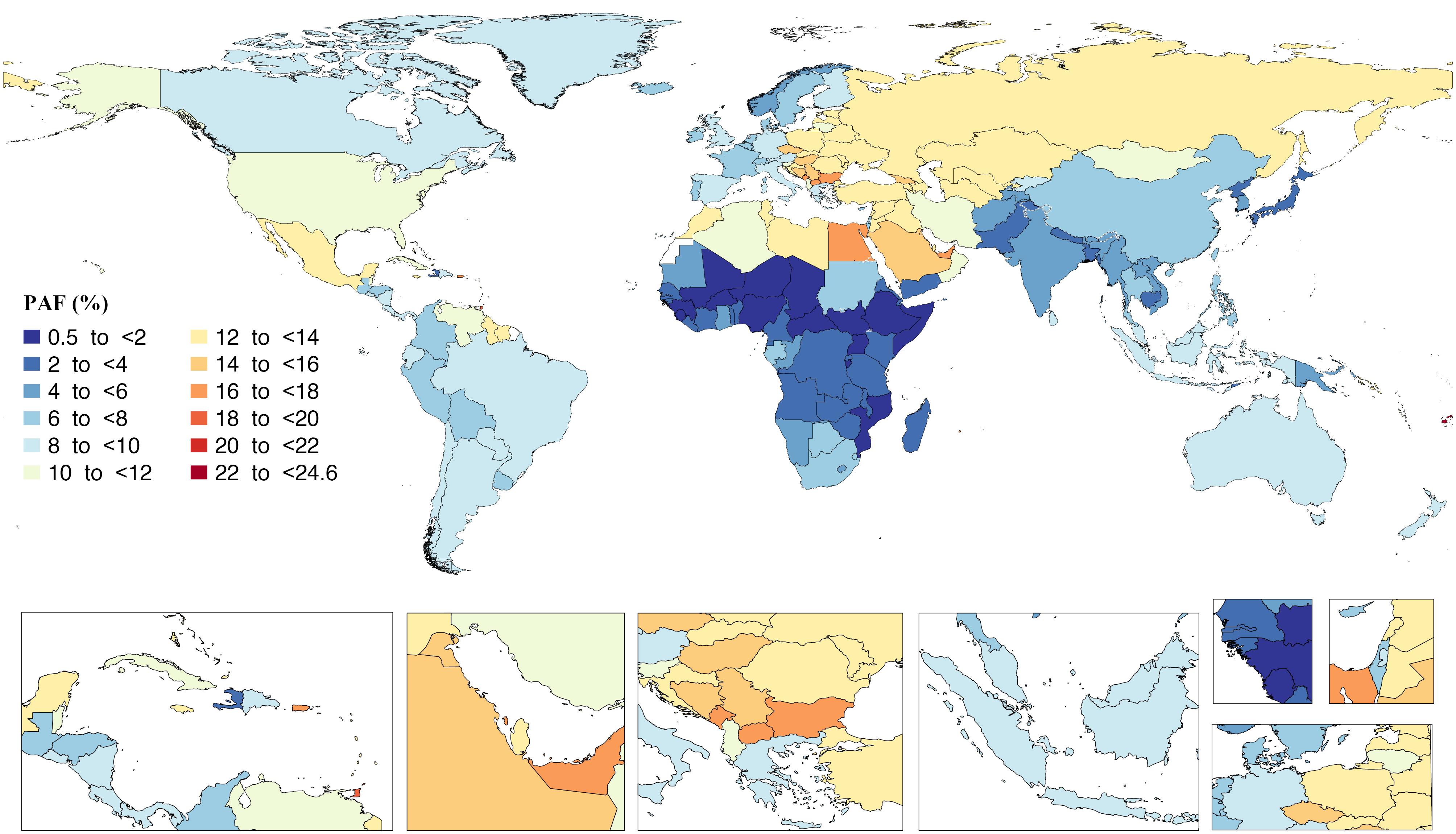
Figure 2.
Population Attributable Fraction of the DALYs From Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available on http://ghdx.healthdata.org/gbd-results-tool
.
Population Attributable Fraction of the DALYs From Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available on http://ghdx.healthdata.org/gbd-results-tool
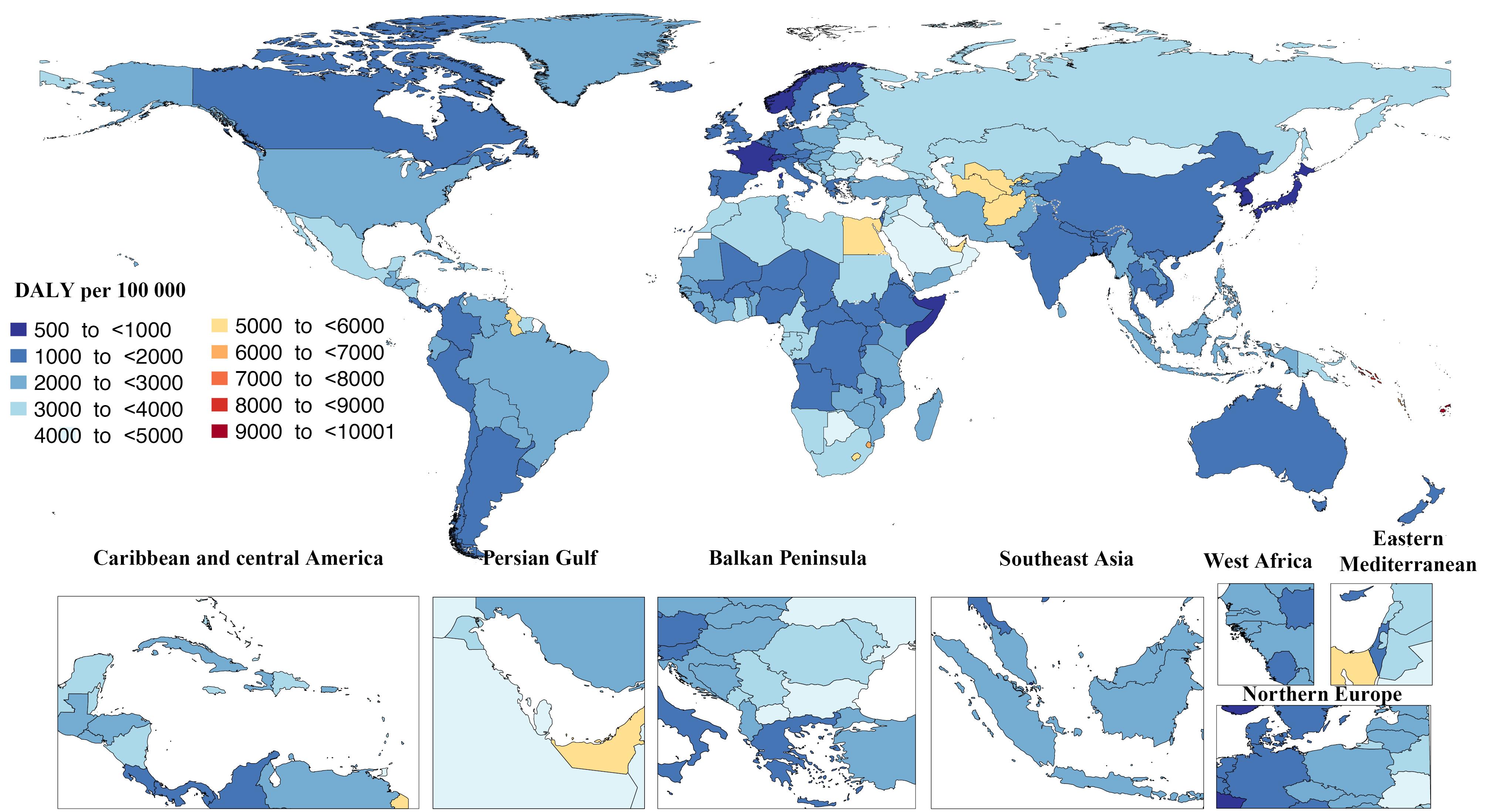
Figure 3.
Age-standardized Rates of the DALYs From Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available from http://ghdx.healthdata.org/gbd-results-tool
.
Age-standardized Rates of the DALYs From Diseases Attributable to High Body Mass Index in 2019 by Country. Note. DALY: Disability-adjusted life years. Source. Generated from data available from http://ghdx.healthdata.org/gbd-results-tool
The death percentage of high BMI in all ages ranged from 1.1 to 31.7. In 2019, Fiji had the highest percentage, with 31.7 (23.3–39), whereas Somalia had the lowest percentage, with 1.1 (0.3–2.4) per 100,000 population (Figure 4).46 In addition, Fiji and Nauru had the highest age-standardized death rate due to increased BMI, with 319.07 (213.7–444.9) and 302.6 (192.9–428.9) per 100 000 population. Contrarily, Japan and the Republic of Korea had the lowest age-standardized rate of death, with 12.6 (4.7–23.6) and 22.8 (10.3–37.6) per 100 000 population (Figure 5).46
Based on the Global Burden of Disease 2017 report, in adults aged ≥ 60 years, the burden of overweight and obesity on mortality and DALYs became increasingly significant. Overweight and obesity-related deaths and DALYs show substantial increases with advancing age, with notable differences between genders.
Among older females, the number of deaths attributable to overweight and obesity peaks in the 75–79 age group, while in males, this peak occurs earlier, in the 65–69 age group, suggesting that males experience the highest mortality burden from overweight and obesity at a younger age compared to females. However, in individuals aged 70 years and older, females surpass males in the number of deaths attributable to overweight and obesity, reversing the trend observed in younger age groups where males have higher death rates. This shift indicates that older women are more susceptible to the cumulative effects of overweight and obesity in later life.
In terms of DALYs, the burden peaks in the 60–64 age group for both genders. However, among those aged 75 years and older, females experience higher DALY rates than males, underscoring the heavier disease burden borne by older women. Notably, a decline in DALYs is observed in the 75–84 age group, which deviates from the upward trend observed in the 60–74 age range. This reduction may be influenced by survivorship bias, where individuals with overweight and obesity may not survive into the oldest age groups, or by the presence of competing health risks.
Despite the considerable increase in absolute numbers of deaths and DALYs among adults aged 60 years and older, age-standardized rates provide a nuanced view. The age-standardized rate of overweight and obesity-related deaths remained stable for females while increasing by 14.5% for males. Meanwhile, the age-standardized DALY rate rose by 12.7% and 26.8% for females and males, respectively, highlighting a more pronounced rise in the disease burden for older men. These findings demonstrate the profound and growing impact of overweight and obesity in older adults, particularly women aged ≥ 75 years. As life expectancy increases in many regions, the long-term health consequences of overweight and obesity in this age group present a significant public health challenge, necessitating targeted interventions to address this growing issue.47,48 The burden of overweight and obesity on mortality and DALYs has significantly increased, particularly in adults aged ≥ 60, with the most severe impact observed in women aged ≥ 75, highlighting a growing public health challenge and a notably greater rate of increase in older adults compared to those ≤ 60.47
The WHO reports that the number of overweight individuals has been rising gradually over the last few decades, and this trend is expected to continue.49 Several different factors influence the prevalence of obesity,50 among which socioeconomic changes, increased access to high-calorie meals, more diverse leisure activities, and changes in work patterns are of importance, leading to a more sedentary lifestyle.50 The development of technology and expanded access to cars, computers, and other devices also contribute to reduced physical activity.51 It is important to note that increased psychological disorders such as anxiety,52 depression, and stress 53 in recent years have contributed to heightened obesity rates through emotional eating, such as bulimia nervosa, and diminished motivation for physical activity.54 The prevalence of psychological disorders has significantly increased in recent decades and is projected to continue rising over the upcoming years. Furthermore, weight gain could be a side effect of certain medications, such as corticosteroids55 and antidepressants.56 Moreover, environmental factors widely influence obesity through the availability of nutritious food alternatives and safe exercise areas, which low-income countries may lack. Further, cultures play a role in influencing people’s food consumption and levels of physical activity.57
Despite the large number of parameters contributing to the increased prevalence of obesity in recent years, as mentioned above, there are preventive measures that can be taken to halt this trend.58 Public health interventions, such as raising public awareness through education campaigns to alter cultural perceptions about obesity and health, are critical in this respect.58 Additionally, policy interventions should be implemented, such as restricting the availability of unhealthy foods and investing in infrastructure such as bike lanes.14 Personalized medicine, tailoring diets to individuals’ cultural customs and genetic predispositions to obesity, offers another powerful tool in obesity management.59 In addition, technology can have a significant role in controlling obesity through smartphone applications and wearable devices. With these tools, monitoring physical activity and providing beneficial advice remotely become feasible.60
Overall, various underlying factors will likely contribute to the future of obesity, exerting both aggravating and preventive effects depending on how their implementation is effective. While anticipating the exact course of events remains challenging, it is evident that without efficient prevention and treatments, the incidence of obesity will likely continue to rise.
Risk Factors of Obesity
Genetic and Epigenetic Factors
Building on traditional methods, current genetic technologies such as genome-wide association studies (GWAS), candidate gene analysis, and next-generation sequencing have identified susceptibility genes implicated in obesity development.61 Research indicates that genetic factors contribute to 40%–80% of obesity,62 with heritability varying according to weight ranges of 30%–35% and 60%–80% in normal-weight individuals and obese and severely obese individuals, respectively. Similarly, heritability estimates across populations are in the range of 40%–80%, 30%–60% for waist-to-hip ratio, and 35–85% for other obesity-related characteristics.63-66 While genetics explains 15% of metabolic syndrome (MetS) according to GWAS analysis, the remaining 85% is attributable to environmental factors.67 Therefore, genetic predisposition is not solely responsible for obesity; its interaction with environmental factors (G × E) plays a significant role.68
Although there is a continuum of clinical characteristics present in genetic forms of obesity, they have historically been categorized into syndromic, non-syndromic monogenic, and non-syndromic polygenic subtypes.69 Monogenic variants of obesity, which are autosomal or X-linked and are inherited according to the Mendelian principle, are characterized by chromosomal abnormalities and uncommon pathogenic mutations in genes that encode critical proteins for the regulation of energy balance.70
Studies demonstrate that childhood obesity, both syndromic and non-syndromic subtypes, occurs on a spectrum. GWAS has uncovered single-nucleotide polymorphisms (SNPs) in or adjacent to syndromic obesity genes such as BDNF, NTRK2, SIM1, BBS2, BBS4, SH2B1, and SDCCAG8.71 Numerous loci associated with non-syndromic genetic childhood obesity, including proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), and the melanocortin-4 receptor (MC4R), have also been linked to polygenic childhood obesity.72 Recent multi-level genetic analyses, including single-marker, tag-SNP, and gene-based approaches, discovered that 17 out of 54 candidate genes for syndromic childhood obesity overlapped with genes and pathways implicated in polygenic obesity, suggesting common mechanisms between these seemingly distinct forms of obesity.73
Syndromic obesity occurs alongside additional clinical symptoms such as developmental delays, skeletal abnormalities, or endocrine problems and accounts for up to 10% of severe obesity cases worldwide. Prader–Willi, Bardet–Biedl, and Alström syndrome are among the common examples of syndromic obesity.74
Monogenic obesity, on the other hand, can be characterized by a single gene mutation that regulates body weight. Given the wide variability of factors contributing to obesity, rare genetic mutations typically cause early-onset severe obesity ( < 10 years old).61 The majority of genes linked to this kind of pediatric obesity are involved in the leptin-melanocortin signaling pathway. AgRP (Agouti-related peptide), PYY (Peptide YY), and MC4R (the melanocortin-4 receptor), among other genes associated with monogenic obesity, interact with hormones that regulate ghrelin, leptin, and insulin, which are sensed by receptors in the hypothalamic arcuate nucleus.75 Consanguinity in populations has enhanced the chance of discovering mutations owing to a higher number of deleterious mutations, as monogenic obesity frequently exhibits a recessive inheritance pattern.76 According to an investigation, mutations in the leptin, LEPR (leptin receptor), and MC4R genes account for 30% of cases of extreme obesity in a consanguineous Pakistani group,77 whereas single-gene abnormalities account for almost 50% of cases.78 Farooqi et al demonstrated that homozygous obesity leptin gene deletion of G133 caused a frame-shift mutation, resulting in a non-secreted truncated protein and severe early-onset obesity, whereas other homozygous mutations result in lower amounts of leptin in circulation.79,80 Similarly, leptin receptor deficiency, an autosomal recessive condition, can occasionally result from a LEPR-splicing mutation that prevents the formation of the transmembrane region.81 It was shown that although obese people had high amounts of leptin, there was also a decline in the number of soluble leptin receptors, which were crucial for leptin activity. Hyperinsulinemia and extreme hyperphagia, together with aggressive behavior when deprived of food, are symptoms of LEP and LEPR deficiencies.75 Among various genetic obesity forms, MC4R mutations are thought to be the most common monogenic cause of severe, early-onset dominance-inherited obesity.82 Pathogenic MC4R mutant carriers have severe early-onset obesity, hyperinsulinemia with euglycemia, a higher frequency of MetS (up to 68%), a higher usage of antihypertensive medications, and frequent binge eating disorder.83 Children with MC4R mutations have a more severe phenotype than adults, as observed by increased hunger, poor satiety, and severe hyperinsulinemia, with the severity of these symptoms decreasing with age.84 Furthermore, POMC mutations and hypermethylation result in a deficiency of the alpha-melanocyte-stimulating hormone, which lowers calorie intake by interacting with MC4R in the hypothalamus.85 Although some studies have linked POMC mutations to glucose metabolism changes, Potoczna et al identified POMC mutations in 48% of individuals with obesity seeking gastric band surgery (13 different variants). However, these mutations did not appear to directly cause obesity or metabolic issues.86,87 Additionally, PCSK1 encodes proprotein convertase 1, a neuroendocrine enzyme that degrades POMC. The absence of this enzyme impairs the normal functioning of other prohormones and neuropeptides, including POMC, leading to early-onset obesity, moderate hyperphagia, and postprandial hypoglycemia.69
Polygenic obesity, also known as common obesity, results from the combined influence of hundreds of small-effect genetic variations and environmental factors. Fat mass and obesity-associated gene (FTO) mutations are the most common causes of polygenic obesity, accounting for 1% of BMI variations in the general population. In addition, recent research has revealed that specific genes are involved in obesity and related health conditions,88 including BDNF, MC4R, and NEGR, which regulate appetite and satiety. Other genes, such as TCF7L2 and IRS1, affect insulin secretion and action, while FTO, RPTOR, and MAP2K5 influence energy and lipid metabolism.88 Gene ontology investigations have also identified shared pathways among obesity-related disorders such as diabetes, HTN, and coronary artery diseases.89 Interestingly, not only do rare genetic variants cause non-syndromic monogenic obesity, but there are also common genetic variations that contribute to polygenic obesity in a variety of ethnic groups. Specifically, several polymorphisms in PCSK1, MC4R, and POMC have been strongly linked to polygenic obesity.90,91
Through the process of epigenetics, which modifies gene expression without changing the DNA sequence, environmental variables such as diet and lifestyle may influence the tendency to become obese. DNA methylation, histone modifications, and non-coding RNAs are the most prevalent epigenetic alterations that have been widely examined in the context of obesity.92 DNA methylation is a well-studied epigenetic process that involves the addition/removal of methyl groups from the cytosine base 5-carbon position, which occurs primarily at CG positions (CpG loci) that are unevenly scattered around the genome, although it less frequently occurs in the non-CG context.93 Several studies have suggested that DNA methylation of genes involved in obesity plays an essential role in obesity development. In this way, DNA methylation of leptin and adiponectin has been shown to be associated with BMI, waist circumference, and low-density lipoprotein cholesterol (LDL-C) levels.94-97 Similarly, it was found that DNA methylation of genes related to eating behavior had a positive association with BMI for POMC98 but a negative association for NPY99 and MCHR1.100,101 Similar findings have also been obtained for the methylation of genes implicated in lipid metabolism (e.g., LPL, PPARG, SREBF1, and CD36),102-104 glucose metabolism (IRS1 and GF2/H19),105,106 circadian rhythm (BMAL1, PER2, PER3, and CLOCK),107,108 hypoxia (HIF3A),109 and inflammation (LY86 and TNFA).110,111
Histone protein tail modifications affect chromatin structure via altering enhancer and promoter activity, which serve critical functions in modulating metabolic genes in response to environmental stimuli in metabolic organs. Histones can undergo a range of chemical modifications after they have been translated from their genetic code, including acetylation, methylation, phosphorylation, adenosine diphosphate ribosylation, O-GlcNAcylation, and lactylation.112 For example, previous research reported that obese individuals had lower levels of H3 lysine 4 trimethylation (H3K4me3) in their adipose tissue compared to lean individuals. This reduction was more prominent near genes involved in the metabolism of fats and inflammation.113 Another case-control investigation involving women with normal weight and women with obesity revealed a variation in histone deacetylase (HDAC) 2/4/5/6 expression that might be correlated with obesity and the inflammatory processes related to obesity.114
Another epigenetic process implicated in obesity development is related to the function of non-coding RNAs, which are the post-transcriptional regulators of numerous biological and pathological pathways. Differential expression patterns of several non-coding RNAs, including microRNAs, long non-coding RNAs, and circular RNAs, among obese and non-obese subjects in different tissues have been well identified and attributed to the dysregulation of several pathways, including those linked with adipocytokines, calcium signaling, MAPK, FOXO, PI3k/Akt, Wnt-β-catenin, and PPAR signaling pathway.115
Building upon our understanding of genetics and epigenetics in obesity, it is now possible to unlock the potential for more personalized and targeted therapeutic approaches.
Diet
Extensive research has focused on the relationship between dietary patterns and chronic non-communicable diseases.116-118 Even though there are many other causes of obesity, poor diet is a major risk factor. One study identified unhealthy dietary patterns as the primary cause of mortality worldwide, surpassing all other risk factors.119 Unhealthy diets are typically those that are high in sodium, trans-fatty acids, sugar-sweetened beverages (SSBs), and red or processed meat and low in fibers, calcium, vegetables, fruits, whole grains, legumes, nuts and seeds, seafood, healthy fats (polyunsaturated and omega-3 fatty acids), and milk.119
Research on environmental changes to the food system and obesity suggests an association between increased food energy availability and higher energy intake with the global rise in obesity rates. A study conducted by Bleich et al120 investigated alterations in energy availability within the food supply of the members of the Organization for Economic Cooperation and Development from 1961 to 2002. The findings revealed that the escalation in the supply of calories was a significant contributing factor to the rise in obesity rates in most countries. According to the results of the study by Kant and Graubard,121 analyzing data from the National Health and Nutrition Examination Survey (NHANES), there was a rise in both the amount and energy density of food consumed in the United States from 1976 to 1980 (NHANES II) and 1999 to 2002 (NHANES III). Additionally, estimates adjusted from the US food supply indicated that per capita calorie intake among the population increased by over 300 kilocalories from 1985 to 2002.122 People’s dietary preferences are influenced by a variety of cultural, environmental, behavioral, and socioeconomic factors.123 The affordability of added sugars, fats, and refined grains has resulted in an increase in diets that are high in energy-dense, nutrient-poor foods, as opposed to those that consist of whole foods such as lean meats, vegetables, and fruits.124
The United States’ out-of-home consumption of food has also increased since the 1970s.125-127 Similar shifts in eating habits have also been observed in Australia, where fast food and eating out are becoming more popular.128 Due in part to the larger serving sizes, high-energy-density foods, increased variety, and preferred flavor of the foods, eating out may raise the risk of obesity.129-131 Fast food consumption in particular has been associated with poor diet quality and detrimental dietary variables related to obesity, including greater intakes of calories, saturated fat, and SSBs.132,133 The consumption of fast food has been linked to an elevated BMI, increased weight gain, and reduced efficacy in maintaining weight loss.132,134-136
The timing of food consumption is another modifiable behavior that may affect how the body regulates its energy and the likelihood of becoming obese. Despite the fact that morning energy intake was irresponsible for obesity, Wang et al137 found that people who consumed ≥ 33% of their daily energy in the evening had a two-fold higher risk of being obese than those who ate in the morning.137 The study findings suggest that a decreased likelihood of being overweight or obese is associated with a higher proportion of daily energy consumption during the midday meal. The combination of an individual’s choices and behaviors, which have an impact on their balance of energy intake, and their genetic and metabolic factors collectively determine their body weight and composition.138
Physical Activity
Physical activity is a crucial modifiable risk factor for obesity.139-141 It refers to any bodily movement produced by skeletal muscles that requires energy expenditure142 and encompasses a wide range of activities, including exercise, sports, and recreational pursuits. Various international health organizations provide guidelines for physical activity to promote health and prevent obesity. For instance, the WHO recommends at least 150–300 minutes of moderate aerobic activity per week (or equivalent vigorous activity) for all adults and an average of 60 minutes of moderate aerobic physical activity per day for children and adolescents.143 Nonetheless, according to the WHO, more than 80% of adolescents and 27% of adults do not meet these recommended levels of physical activity.143 This affects individuals over their life course and places a financial burden on health services and society.143,144 The WHO’s Global Action Plan on Physical Activity 2018-2030 provided recommendations to help countries increase levels of physical activitywithin their populations. Regular physical activity promotes mental and physical health in people of all ages. It is proven to help prevent and treat non-communicable diseases such as heart disease, stroke, diabetes, and breast and colon cancer.143,144 It also helps prevent HTN, overweight, and obesity and can improve mental health, quality of life, and well-being.143,144
Higher physical activity levels are associated with a lower risk of obesity or weight gain over time.141,145,146 Engaging in regular physical activity has been consistently associated with a reduced risk of obesity and its related health complications.145,147-155 Conversely, insufficient physical activity or a sedentary lifestyle is related to an increased risk of obesity156-158 and weight-related health problems, including metabolic disorders159-161 and CVDs.162-164 Despite the well-established benefits of physical activity, the global prevalence of physical inactivity remains a significant concern.165,166 Sedentary behaviors, such as prolonged sitting and excessive screen time, have become increasingly prevalent due to modern lifestyles and technological advancements.167,168 These sedentary behaviors contribute to reduced physical activity levels and an increased risk of obesity.158
This link can be understood through the concept of energy balance, which is determined by the equilibrium between calories consumed (energy intake) and calories expended (energy expenditure).169 The mechanisms underlying the relationship between physical activity and obesity involve complex physiological processes related to energy expenditure, appetite regulation, and fat distribution.169,170 Regular physical activity increases total energy expenditure through both exercise-induced energy expenditure and non-exercise activity thermogenesis.171,172 Non-exercise activity thermogenesis refers to the energy expended during daily activities such as walking, standing, and fidgeting, which can significantly contribute to overall energy balance.173 Physical activity plays a role in energy expenditure by increasing energy demand, implying that as individuals engage in physical activity, their muscles require additional energy to perform the work.174,175 This energy is derived from the metabolism of stored body fat and glycogen.176-178 Consequently, regular physical activity helps increase total energy expenditure and create a negative energy balance, favoring weight loss or weight maintenance.179 Furthermore, physical activity influences body composition by reducing fat and preserving or increasing lean mass.180,181 Further, it stimulates fat oxidation, promoting the use of stored fat as a source of energy, and enhances post-exercise lipid metabolism, which contributes to weight loss.177-179 Moreover, physical activity helps maintain muscle mass,182 which is more metabolically active than fat tissue and contributes to a higher resting metabolic rate.183,184
Physical activity also affects appetite regulation and hormonal responses. Intense physical activity has been shown to influence various appetite-regulating hormones, such as ghrelin and peptide YY, leading to decreased appetite and improved satiety.185-187 In other words, engaging in physical activity can reduce cravings and emotional eating, helping individuals to maintain a balanced and healthy diet. Physical activity also influences the psychological and behavioral factors related to obesity.188 Regular exercise has been shown to improve mood, reduce stress levels, and enhance overall mental well-being.189,190 It can also be a positive coping mechanism for emotional and stress-related eating, reducing the risk of overeating and weight gain. Furthermore, engaging in physical activity can improve self-esteem and body image, promoting a healthier relationship with one’s body and reducing the risk of disordered eating patterns.191,192 Additionally, physical activity is crucial for long-term weight maintenance. While diet plays a significant role in initial weight loss, sustaining weight loss over time often requires regular physical activity.193
Behavioral and Psychological Factors
Obesity is a complex condition that is influenced by a number of different causes, including psychological and behavioral factors.194 These factors play an essential role in the development and progression of obesity, alongside genetic, environmental, and physiological elements.194,195 It is crucial to understand the psychological and behavioral risk factors associated with obesity to effectively prevent and manage the condition.195
Behavioral treatment has been shown to cause modest weight loss of around 5%–10% of initial body weight.196,197 Psychological factors such as having unrealistic weight goals, poor coping or problem-solving skills, and low self-efficacy can hinder weight maintenance and increase the risk of relapse into obesity.198
Depression and anxiety, two of the most common psychiatric disorders, are strongly associated with obesity.199 Previous research has linked obesity with depression and anxiety,199-202 although some studies suggest that body fat distribution and sleep behavior might play an even greater role in this association.203-205 A systematic review by Luppino et al revealed that people with obesity had a 33% increased risk of developing anxiety over time and that anxious people had an 84% increased risk of developing obesity over time.200 According to another study, people with obesity were 32% more likely than people with a normal BMI to have depression.206
Depression is characterized by persistent feelings of sadness, loss of interest, and a range of emotional and physical symptoms.207 While not all individuals with depression experience changes in appetite or sleep patterns, these symptoms are relatively common.208-210 Depression can lead to increased appetite, particularly for high-calorie comfort foods.211,212 Hormonal and neurochemical changes, such as dysregulated cortisol levels (the stress hormone), can contribute to an increased preference for fat, sugar, and carbohydrate-rich foods.210-212 Depression can also disrupt the functioning of other appetite-regulating hormones such as leptin and ghrelin, leading to overeating and weight gain.213-215
Furthermore, depression often coexists with sleep disturbances such as insomnia or hypersomnia, both of which can influence appetite regulation and energy balance.216 Sleep disturbances can disrupt the normal production and release of hormones that regulate appetite.208,216 Reduced sleep duration or quality can affect leptin and ghrelin levels,208 resulting in increased hunger and cravings for high-calorie foods, thereby contributing to weight gain.217,218 Conversely, excessive sleepiness or hypersomnia can disrupt regular meal schedules, leading to irregular eating patterns and a higher likelihood of consuming larger meals or frequent snacking.209 Excessive sleepiness can also reduce motivation for physical activity, promoting a sedentary lifestyle and weight gain.219
Depression, appetite dysregulation, and sleep disturbances are interconnected, forming a complex interplay that exacerbates the risk of obesity.220 Changes in eating behaviors and disrupted sleep patterns create a cycle that contributes to weight gain. Managing/preventing obesity in individuals with depression requires addressing the psychological symptoms, as well as the associated appetite and sleep changes.
The relationship between anxiety and obesity is complex and involves various psychological, behavioral, and physiological factors.221 While anxiety itself may not directly cause weight gain, the behaviors and physiological responses associated with anxiety can contribute to weight gain and the development of obesity.222,223
Emotional or stress eating is common among individuals experiencing anxiety, as they may turn to food to cope with emotional distress.224 This can lead to excessive calorie intake and weight gain over time. Anxiety can also disrupt normal eating patterns, with some individuals experiencing loss of appetite during heightened anxiety and others resorting to binge eating as a response to anxiety.224 These irregular eating patterns negatively affect metabolism and contribute to weight gain.225
Anxiety triggers the release of stress hormones, such as cortisol, which can affect weight regulation.212,226 Elevated cortisol levels increase appetite, especially for calorie-dense foods, and promote fat storage, particularly in the abdominal area.212,226 This hormonal response contributes to weight gain, particularly in individuals experiencing chronic or prolonged anxiety.212,226 Additionally, anxiety can lead to a sedentary lifestyle and reduced physical activity, as individuals may avoid anxiety-inducing activities or situations, including exercise and social interactions.227 The lack of physical activity decreases energy expenditure and further contributes to weight gain.
Moreover, some individuals with anxiety or depressive disorders may be prescribed medications, such as certain types of antidepressants, which can have weight gain as a side effect.228,229 Not all anxiety medications have this effect, but some antidepressants, such as tricyclic antidepressants,230 and monoamine oxidase inhibitors,231 are more likely to cause weight gain due to their effects on appetite, metabolism, and insulin sensitivity. It is noteworthy that individual responses to antidepressants can vary, and not everyone will experience weight gain or obesity-related effects.
Socioeconomic Factors
Socioeconomic factors significantly influence the prevalence and persistence of obesity.232-234 Income and socio-economic status, food environment and neighborhood characteristics, education, health literacy, and social and cultural factors are all intertwined and play important roles in shaping an individual’s risk of obesity.232-236
Income and socioeconomic status (SES) are among the most critical socio-economic factors influencing obesity.235,236 Several studies have found a strong association between low income and higher obesity rates, particularly in low-income countries.235,236 In countries with a low human development index or low-income countries, there is also a positive correlation between SES and obesity for both men and women, implying that more affluent individuals in low-income countries are more likely to be obese.236 In terms of obesity in children, it seems to be predominantly a problem of the wealthy in low- and middle-income countries.236 Nevertheless, some studies have reported that neighborhood SES had a more robust and more consistent relationship with obesity for women than for men and for higher-income women than for lower-income women.237 Due to their higher costs, individuals with limited financial resources often face challenges accessing healthy food options, such as fresh fruits, vegetables, and lean proteins.238-242 As a result, they are more likely to rely on cheaper, energy-dense processed foods that are high in calories and low in nutritional value.238-240 Additionally, individuals with a lower SES may have limited access to recreational facilities and opportunities for physical activity, further exacerbating the risk of obesity.243
The food environment and neighborhood characteristics also significantly influence obesity rates.244,245 Specific communities, often those with lower SES, may lack access to grocery stores or farmers’ markets offering fresh, affordable, and nutritious foods.246 These areas are described as “food deserts”, where the predominant food options are convenience stores and fast-food outlets, which tend to offer energy-dense, low-quality foods.246 The absence of safe, walkable neighborhoods with parks and recreational spaces limits opportunities for physical activity, reinforcing sedentary behaviors and contributing to obesity.243,247
Education and health literacy are critical socio-economic factors influencing obesity.248 Low levels of education and health literacy can impede individuals’ understanding of nutrition and health-related information, making it challenging to adopt and maintain healthy behaviors.239 Limited health literacy can affect an individual’s ability to navigate food labels, comprehend dietary guidelines, and make informed decisions about their diet and lifestyle.239 Moreover, individuals with lower education levels may face economic constraints that limit their ability to access healthcare services, including preventive measures and weight management programs.249
Social and cultural factors also contribute to obesity rates.250 Peer influence, social norms, and cultural practices can shape individuals’ attitudes toward food and physical activity.250,251 For instance, certain cultural celebrations and traditions may center on large, indulgent meals, leading to excessive calorie consumption.252 Furthermore, social networks and social support play a crucial role in influencing individuals’ eating habits and physical activity levels.253-255 Lack of supportive social networks or living in an environment where unhealthy behaviors are the norm can contribute to developing and maintaining obesity.254,255
Recognizing and addressing these socio-economic determinants of obesity are crucial for developing effective interventions and policies aimed at preventing and managing obesity at the population level, as well as promoting health equity and improving overall well-being.
Health Consequences and Burden of Diseases Attributable to Obesity
Cardiovascular Diseases
Obesity and high BMI have numerous negative impacts on an individual’s health, specifically in relation to the prevalence and severity of CVD and its risk factors, which is the primary cause of death in the United States for the majority of racial and ethnic groups.19,20 This significant comorbidity is regarded as a negative prognostic factor with respect to overall life expectancy, heightened mortality, and morbidity.256 For instance, early childhood obesity (by the ages of 11–12) is positively associated with the emergence of cardiovascular problems.257 It is noteworthy that obesity, independent of other factors, has been found to significantly elevate the risk of nearly all CVD risk factors, such as HTN, glucose abnormalities, including T2DM, MetS, and dyslipidemia, as well as levels of inflammation. Moreover, the condition of obesity has the potential to generate a range of metabolic, neurohormonal, and hemodynamic modifications that could have negative effects on the morphology of the heart and the functioning of its ventricles.258-261 As a result, obesity is strongly associated with an elevated risk of CVDs, particularly heart failure,262 atrial fibrillation, coronary heart disease, HTN, and numerous other forms of CVD.19,262,263
The discovery of leptin and adiponectin in the 1990s sparked a revolution in our understanding of adipose tissue, transforming it from a mere energy bank into a dynamic and multifaceted endocrine organ. This groundbreaking discovery reignited research into the crucial role adipose tissue plays in interorgan communication, opening a new chapter in our exploration of its far-reaching influence on health and disease.264,265
Leptin has contradictory and counterintuitive effects on cardiovascular health. Frequently, hyperleptinemia is strongly associated with adverse outcomes in CVDs.266,267 Leptin, however, can occasionally have cardioprotective effects by lowering cardiomyocyte apoptosis and hypertrophy.268
There is no denying the strong correlation between low levels of adiponectin and the increased prevalence of obesity-related cardiovascular illnesses, such as peripheral artery disease and ischemic heart diseases. Higher values of circulating adiponectin, however, make the issue more complicated. Elevated levels of adiponectin have been found in some circumstances to be correlated with a favorable prognosis for cardiovascular events. Conversely, in some circumstances, increased levels of adiponectin have no positive effect or even unfavorable effects, such as an increase in mortality rate. This phenomenon is known as the “adiponectin paradox”.269,270
The escalating worldwide prevalence of obesity has been accompanied by a corresponding increase in the incidence of sleep disorders that are associated with respiratory difficulties, specifically those that manifest as repeated pharyngeal airway collapses during sleep.271 While obesity may be a contributing factor to the development or exacerbation of sleep apnea, it is important to note that this condition can also have negative impacts on cardiovascular health. OSA is considered the most prominent sleep disorder due to its association with diverse CVDs and metabolic comorbidities.272,273 Sleep disorders have been thought to play a causal role in the pathogenesis of weight gain, while obesity is widely recognized as the most influential demographic risk factor for the onset and advancement of OSA.271,272
Several researchers have concluded that the risk of all-cause mortality increases with prolonged obesity duration, regardless of various potential confounding factors and even independent of current BMI. This correlation demonstrates a particularly strong association with cardiovascular disease mortality.274,275 Conversely, other researchers have found evidence that current BMI is a stronger predictor than the duration of obesity.276,277
Neoplasms
Obesity has been identified as an independent risk factor for a variety of neoplasms, with endometrial, postmenopausal breast, and colorectal cancers accounting for more than 60% of obesity-related malignancies.278 Based on the population-attributable fraction estimation of cancer incidence for 12 cancer types in 175 countries and 9 global regions, it was determined that in 2012, there were approximately 544 300 cancer cases—or 3.9% of all cancer cases worldwide—that could be linked to excess body weight in 2002.279 Another study involving prospective data from 3,850 subjects concluded that weight gain of more than one pound per year was correlated with a 38% increase in overall cancer risk, with women having the highest risk.280
Specifically, obesity has been associated with an increased risk of estrogen receptor-positive and triple-negative phenotypes of postmenopausal breast cancer.281,282 However, in the premenopausal period, the relationship between obesity and cancer incidence was found to be less linear and even negatively correlated with the risk of breast cancer. Individuals with a BMI of more than 35 kg/m2 had a 76% lower risk of premenopausal breast cancer than those with a BMI of less than 17 kg/m2.278,283 Similarly, recent meta-analyses have indicated that obese women with breast cancer face a 30% higher risk of recurrence or mortality than normal-weight women.284,285
According to studies on the burden of colorectal cancer in obese individuals, higher weight circumference was a stronger risk factor than BMI and was linked to a higher risk of colorectal cancer, with each 10 cm increase in weight circumference leading to a 4% increase in colorectal cancer risk.286 Furthermore, it has been established that this risk was greater in males than in females and that it was greater in elderly women above the age of 70 than in younger women.287 Evidence from the past several decades has revealed a strong correlation between the increasing incidence of early-onset colorectal cancers and the childhood and adolescent obesity epidemic.4 In a study involving a cohort primarily consisting of white women in the United States, individuals with a BMI of 23, compared to those with a BMI of 18.5–20.9 at the same age, had an almost 60% higher risk of developing early-onset colorectal cancer.288
Endometrial cancer has been found to be four times more common in obese women than in non-obese women. Survivors with a BMI greater than 30 had a 2.28 relative risk of mortality compared to those with a BMI less than 22.5.289,290 Similarly, another retrospective study on endometrial cancer indicated a positive correlation between obesity and the grade and stage of the disease.291 In addition, for esophageal adenocarcinoma, gallbladder, kidney, liver, multiple myeloma, ovary, pancreas, stomach cardia, and thyroid cancers, each 5-unit increase in BMI was associated with a 48%, 25%, 30%, 30%, 12%, 6%, 10%, 27%, and 13% increased risk of cancer development, respectively.292
Diabetes and Kidney Diseases
The correlation between obesity and the onset of T2DM is widely acknowledged due to the heightened insulin resistance associated with obesity. Insulin resistance serves as a predisposing factor for T2DM.293 Early research294 revealed that the most significant environmental factor affecting the prevalence of diabetes in a population was the level of obesity. Excess weight is responsible for 90% of T2DM cases.295 According to several studies, individuals with BMIs of 30–35 kg/m2 had a greater than 20-fold increased risk of developing diabetes in women and a 10-fold increased risk in men.296,297 In addition, it is noteworthy that the presence of adipose tissue in the upper body or its central distribution constitutes a significant risk factor for the development of T2DM, independent of the individual’s overall obesity level.298-301
The occurrence of T2DM is attributed to the pancreas’ inability to compensate for the decline in insulin sensitivity.302 This phenomenon is particularly prevalent in individuals with obesity, who exhibit higher levels of non-esterified fatty acids, glycerol, and pro-inflammatory cytokines originating from adipose tissue.303 Hormones such as resistin, leptin, and adiponectin, among others, also play crucial roles in insulin resistance and the development of T2DM in individuals with obesity.304,305
Obesity significantly contributes to the pathogenesis of T2DM through various pathways. Obesity exacerbates the mortality rate and increases the risk of microvascular complications in diabetic patients.306,307 Furthermore, obesity worsens the prognosis of T2DM.308 Particularly concerning is the observation that obese individuals with diabetes face a poorer prognosis in terms of long-term disease outcomes compared to their non-obese counterparts. This trend is alarming, considering the global escalation in the prevalence of obesity and T2DM.309-311
Obesity in type 1 diabetes (T1DM), which was formerly uncommon, is now a growing problem.312-314 There is mounting evidence that people with T1DM are also affected by obesity-related comorbidities, which were previously thought to be more common in people with T2DM. Overweight and obesity can contribute to insulin resistance in those with T1DM.315 The prevalence and effects of obesity in T1DM are becoming increasingly obvious due to mounting evidence, which demonstrates how poorly recognized and undervalued this comorbidity is.316
In addition to T1DM and T2DM, obesity significantly raises the risk of developing gestational DM (GDM). Research indicates a positive correlation between BMI increase and the likelihood of developing GDM.317 Both GDM and obesity are associated with alterations in adipokine levels, which affect normal lipid and glucose metabolism. Insulin resistance-induced changes in glucose metabolism lead to elevated levels of free fatty acids in the blood, resulting in lipotoxicity.318 Common features of GDM and obesity include lipotoxicity, glucotoxicity, and hyperinsulinemia, collectively contributing to increased oxidative stress. Maternal and fetal circulatory antioxidant mechanisms help mitigate these modifications.318
The development and progression of chronic kidney disease (CKD) have been shown to be correlated with several obesity-related variables in numerous population-based studies. In those without renal disease, a higher BMI has been linked to the development and presence of proteinuria.319-323 Moreover, several extensive population-based studies have indicated a correlation between elevated BMI and the existence and progression of a low estimated glomerular filtration rate,319-321,324-325 a faster decline in the estimated glomerular filtration rate over time,326 and the occurrence of end-stage renal disease.327-330 In individuals with pre-existing CKD, elevated BMI levels and class II obesity and above have been associated with a faster rate of CKD progression.331
Obesity-related kidney disorders are histologically categorized into glomerular and renal tubular injuries.332 Among these, obesity-related glomerulopathy (ORG) stands out as a condition uniquely affecting individuals with obesity, representing one of the most prominent chronic renal consequences of obesity. ORG is defined as a renal disease characterized by proteinuria in individuals with a BMI of 30 kg/m2 or higher, occurring in the absence of clinical and histopathological findings of other renal diseases.333
Between 1986 and 2015, the prevalence of obesity-associated glomerulopathy grew more than tenfold, coinciding with a higher average BMI among ORG patients compared to the general population.334 Early studies revealed that patients with obesity-induced proteinuria exhibit focal segmental glomerulosclerosis lesions and glomerular hypertrophy upon renal biopsy.335 Animal experiments have consistently demonstrated that obesity induces renal tubular damage, characterized by tubular hypertrophy, fibrosis, tubulo-interstitial inflammation, and the formation of lipid cytoplasmic inclusions.336,337 Furthermore, obesity-related hyperfiltration appears to significantly contribute to the development of tubular injury, akin to its role in diabetic nephropathy.338
Neurological Disorders
Extensive research has explored the relationship between obesity and its effects on brain structure and cognitive abilities. Obesity has been strongly correlated with mild cognitive impairment and an increased risk of AD. Studies indicate that obesity may influence cognitive functions, leading to changes in memory, learning, and other cognitive processes.339
Numerous experimental studies employing animal models of high-fat diet (HFD)-induced obesity have revealed significant findings. Specifically, the hippocampus, a crucial brain region for memory and learning, has been shown to undergo structural and functional changes in response to obesity. Obesity-associated cognitive deficits have been linked to hippocampal alterations. Additionally, individuals with obesity exhibit cortical atrophy, particularly in the context of AD.340 Moreover, several experimental studies have investigated markers of AD-related pathology in rodents fed HFDs. The HFD-fed mice demonstrated increased expression of amyloid precursor protein and amyloid precursor protein-processing enzyme, alongside abnormalities in learning and memory as well as reduced executive function, consistent with clinical observations. Notably, long-term potentiation, a key mechanism supporting learning and memory, was compromised in mouse models fed an HFD. Additionally, consumption of an HFD has been reported to decrease the brain-derived neurotrophic factor and other markers associated with neurogenesis, synaptic plasticity, and neuronal development. Intriguingly, numerous studies using animal models have produced compelling evidence, suggesting that hypothalamic inflammation precedes a vicious cycle of central nervous system dysfunction, ultimately leading to cognitive decline.341 Furthermore, vascular dementia, which is often caused by cerebrovascular disease, can be exacerbated by obesity. These effects include the promotion of atherosclerosis in large cerebral arteries and alterations in the cerebral microcirculation.342 T2DM has also been linked to accelerated cognitive decline and an increased risk of dementia. Shared metabolic, oxidative, and inflammatory processes are thought to contribute to neurodegeneration in both obesity and T2DM-related conditions.343
The concept of the “obesity paradox” arises from observations that obesity is associated with improved survival rates in certain conditions, such as heart failure and stroke. However, the underlying mechanisms behind this phenomenon remain incompletely understood. Further research is warranted to elucidate the specific factors contributing to these outcomes.344 In conclusion, obesity profoundly affects cognitive function, brain structure, and various neurological disorders. It correlates with cognitive impairment, changes in the hippocampus and cortex, disruptions in the autonomic nervous system, and heightened risk of central disorders. Further research is needed to fully understand the underlying mechanisms and identify potential therapeutic targets linked to these relationships.
Chronic Respiratory Diseases
Obesity can influence respiratory conditions such as asthma, exertional dyspnea, OSA, obesity hypoventilation syndrome, and chronic obstructive pulmonary disease (COPD), serving as both a preventable risk factor and a disease modifier.345-348 Furthermore, obesity adversely impacts outcomes for acute respiratory distress syndrome and COPD, as well as increasing vulnerability to respiratory infections. Hospitalization rates are also elevated in obese patients with respiratory diseases in comparison to those with a healthy weight.347 Both adults and children can experience wheezing, dyspnea, airway hyperresponsiveness, and orthopnea due to obesity, which can affect the prevalence and severity of the lung diseases mentioned earlier.347,349,350
Obesity exerts diverse effects on the respiratory system, including alterations in lung capacity and disruptions in cytokines and adipokines. Adipose tissue generates over fifty different adipokines, which can foster inflammation in conditions such as asthma and COPD. Normally, adipokines help maintain lung health through balancing pro- and anti-inflammatory actions.351-353 However, obesity disrupts this equilibrium, leading to the onset and progression of lung disorders.354 Additionally, surplus fat on the chest wall can reduce the compliance and endurance of respiratory muscles, resulting in increased breathing efforts and airway resistance.355
Weight gain and an increasing BMI can lead to reduced lung volumes, resulting in a more restrictive ventilation pattern on spirometry. An elevated BMI is associated with decreases in forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), functional residual capacity, and expiratory reserve volume (ERV) in both cross-sectional and longitudinal studies. Individuals with morbid obesity (BMI > 40 kg/m2) may experience a slight reduction in residual volume (RV) and total lung capacity, while functional residual capacity may approach RV at this level of obesity.345,356-361 The relationship between BMI and FEV1 is intricate; FEV1 declines at a slower rate compared to FVC or ERV. Additionally, the correlation between an increase in BMI and a decrease in FVC is more pronounced than that with FEV1.
Obesity is commonly linked to a restrictive lung ventilatory defect rather than an obstructive one, characterized by a low FVC and a high FEV1/FVC ratio ( > 70).358,361-362 Patients with COPD exhibit a higher prevalence of obesity compared to those without the condition, underscoring the association between obesity and certain chronic respiratory diseases.363 The obesity paradox is frequently observed in individuals with COPD.364 While being overweight is typically associated with increased mortality risk, a significant percentage of overweight or obese individuals have been shown to have a higher chance of survival compared to those with lower body weight, a phenomenon referred to as the obesity paradox.365-367
The relationship between obesity and asthma is well-established, with a meta-analysis of over 300 000 adults finding that increasing BMI was associated with an increased risk of asthma.368 Obesity is a significant risk factor for the development of asthma and is often linked with more severe forms of the condition.369 Research indicates that obese individuals with asthma experience higher rates of asthma exacerbations.370 They further tend to have more severe disease manifestations, exhibit reduced responsiveness to standard therapies, and may demonstrate cellular glucocorticoid resistance.355,370,371 Consequently, obesity can contribute to the development of more severe asthma phenotypes and associated comorbidities.372,373
Sleep apnea is a prevalent respiratory disorder that affects a significant portion of the population.374 Obesity is the most significant risk factor associated with sleep apnea, and the relationship between the two is particularly concerning given the global obesity epidemic. The economic costs of sleep apnea are substantial for both the individual and society as a whole. Specifically, sleep apnea contributes to significant economic burdens related to motor vehicle collisions. Moreover, individuals with sleep apnea often experience daytime sleepiness, reduced quality of life, and impaired cognitive function, including deficits in learning skills, episodic memory, executive function, attention, and visuospatial cognitive functions. Left untreated, sleep apnea can lead to a range of medical complications, including CVDs, which can further increase healthcare utilization.374
A higher BMI greatly increases the likelihood of developing OSA.375 Increased fat deposition around the soft tissues of the neck and tongue, often caused by obesity, can significantly contribute to upper airway obstruction. This can lead to an increase in extra-luminal pressures in the pharynx, which can elevate critical closing pressure and ultimately increase the likelihood of airway collapse.376 In addition, it has been demonstrated that higher levels of obesity can exacerbate nocturnal hypoxemia in individuals with OSA.377 To sum up, obesity has complex impacts on respiratory health and conditions, including worsening preexisting lung issues, increasing the chances of developing respiratory problems, and changing the way our bodies react to treatments.
Digestive Diseases
The interaction between the gastrointestinal (GI) tract and fat tissue occurs under both healthy and pathological circumstances. This interaction pathway presents an opportunity to discover novel therapeutic methods for obesity or GI diseases, which is consistent with previous evidence, suggesting the significance of peripheral signals in controlling GI motor responses and regulating food intake. Numerous pathways, including mechanical, pro-tumoral, pro-cancerogenic, and nutritional factors, may link obesity with GI diseases.378 The quantitative and qualitative changes in gut microbiota observed in obese patients are associated with several pathophysiological pathways, potentially explaining the connection between GI diseases and obesity. Numerous investigations have demonstrated a relationship between intestinal dysbiosis and obesity.379
Obese individuals typically exhibit less diversified microbiomes and a different ratio of the two main gut phyla, Bacteroidetes and Firmicutes. This imbalance results in a microbiota with an enhanced capacity for dietary energy harvesting, characterized by an increase in Firmicutes and a decrease in Bacteroidetes. Another consequence of obesity-associated dysbiosis is the reduced production of anti-inflammatory short-chain fatty acids, particularly butyrate, which also possesses anti-tumor properties through the regulation of different G-protein-coupled receptors and the suppression of histone deacetylases. These changes in microbiome diversity and metabolites can lead to increased leaking of intestinal antigens, such as lipopolysaccharides from Gram-negative bacteria, resulting in metabolic endotoxemia. This condition contributes to the generation of procarcinogenic toxic metabolites and increased extraction of energy and nutrient availability, leading to metabolic dysregulation that promotes tumor growth.380 Therefore, the gut microbiota may serve as a key link between obesity and cancer. The prognosis for GI cancer is poorer in obese individuals, with numerous factors contributing to the correlations with neoplasia. Barrett’s esophagus and gallstones, two well-established cancer-related diseases, have a higher prevalence in overweight individuals.
Obesity and MetS are also associated with preneoplastic lesions, including pancreatic intraepithelial neoplasia, colorectal adenoma, and serrated lesions of the colon. Adipocytes can release cancer-causing substances such as adipokines, insulin-like growth factors, and vascular endothelial growth factors at the cellular level. Another potential source of carcinogens is inflammatory cells, which are more prevalent in adipose tissue and systemically circulate in obese people. Animal research has provided further insights.381,382 For example, intestinal adenomas were larger and more common in mice fed an HFD with a genetic predisposition to colorectal cancer compared to the control mice, and higher levels of mucosal oncogenic markers may be present. While weak-to-moderate correlations have been found between GI illnesses and obesity, and the underlying mechanisms are not fully understood, it is evident that obesity and multiple sclerosis have a significant, often overlooked, and potentially preventable impact on the prevalence of GI disease.383
Additionally, emerging mediators of the link between obesity and GI cancer include intestinal hormones. For instance, the fundic glands of the stomach produce the peptide hormone ghrelin, which possesses several metabolic and inflammatory properties, including appetite regulation, fat storage promotion, and inhibition of pro-inflammatory cytokine development. Individuals with obesity have been observed to have lower levels of circulating ghrelin than those with normal BMIs. According to a case-control study involving patients with esophageal adenocarcinoma and patients with non-cardia stomach cancer, the lowest quartile of ghrelin levels was associated with an approximately fivefold higher risk of developing both types of cancer when compared to the highest ghrelin level category.384
Musculoskeletal Disorders
The musculoskeletal system is crucial for facilitating daily activities and leisure pursuits. However, deviations from its standard structure or arrangement can impede functionality, leading to discomfort, pain, and sometimes physical incapacity in certain instances.385
Obesity has been associated with various chronic musculoskeletal conditions, including fibromyalgia,386 osteoarthritis,387 osteoporosis,388 inflammatory arthritis,388 gout,389 chronic low back pain, carpal tunnel syndrome,390 lumbar spine and soft tissue conditions,388 and pelvic pain. 391,392 Numerous studies have highlighted the adverse effects of obesity on the morphology and physiology of the musculoskeletal system, particularly in the lower extremities and feet. For example, research indicates that individuals with obesity, irrespective of age, tend to have wider, thicker, and flatter feet than those without obesity.393-397 Additionally, obese individuals exhibit noticeably higher plantar pressures while standing and walking398-403 and undergo biomechanics changes in their feet and lower limbs while walking404-406 compared to people without obesity. The development of musculoskeletal pain and discomfort in the foot has been linked to the continuous burden of carrying excess body mass among overweight and obese individuals.407-409 Furthermore, being overweight or obese increases the likelihood of developing foot and ankle tendinitis, plantar fasciitis, chronic pain in one or more lower extremity regions, decreased mobility, and persistent plantar heel discomfort.410-412 Overweight and obese individuals are also more prone than healthy or underweight individuals to experience chronic pain.413 Further, obese individuals have a four-time higher prevalence of knee osteoarthritis than non-obese individuals.414 Additionally, a retrospective case-control study 415 revealed an association between BMI and Meralgia paresthetica, a sensory mononeuropathy affecting the lateral femoral cutaneous nerve. Observational studies suggest a positive correlation between high BMI and the presence of degenerative disc disease of the lumbar spine, as detected through MRI.416 Steele et al found that obesity compromises kyphosis and thoracic spine loading in the upper body of women. Their study indicated a positive correlation between elevated obesity rates and heightened occurrences of musculoskeletal pain in the upper torso region.417
Sense Organ Diseases
Obesity is associated with an imbalance between the sympathetic and parasympathetic divisions of the autonomic nervous system.418 Increased sympathetic outflow to neuro-adipose junctions stimulates lipolysis via stimulatory G-protein-coupled β-adrenoceptors. This triggers a downstream signaling pathway that ends with the activation of adipose triglyceride lipase, resulting in triglyceride hydrolysis. Thus, sympathetic hyperactivation, as observed in obesity, can promote a feed-forward mechanism leading to an increased pool of circulating long-chain fatty acids.419 Several investigations have highlighted obesity as a standalone risk factor for polyneuropathy. Recent evidence has shown an increased risk of polyneuropathy with the number of MetS components, independent of glycemic management.420 Abnormal lipid profiles, frequently observed in early T2DM, are associated with the onset of polyneuropathy. Increased triglyceride levels, in particular, have been linked to the development of polyneuropathy in diabetes. Additionally, individuals with prediabetes, who are typically obese, and patients with idiopathic polyneuropathy have noticeably higher triglyceride levels. These findings suggest that obesity and dyslipidemia may contribute to the pathogenesis of polyneuropathy.421 Moreover, weight and chronic pain have been found to be significantly correlated with higher rates of pain complaints and diagnoses detected in individuals with higher BMIs. Furthermore, a higher body fat percentage and the presence of MetS indicators have been related to clinical pain situations. Studies on spinal cord injury have indicated that individuals who are overweight or underweight experience more severe pain than those of normal weight. However, further study is required to examine the connection between obesity and chronic pain in people with spinal cord injury, particularly based on the recently adopted definition of obesity for this population.422
Psychological Issues
Although early studies did not identify variations in the overall psychological state between individuals with obesity and those without,423 more recent investigations have revealed that obesity is associated with multiple psychological problems. According to several studies, between 20% and 60% of individuals with obesity, particularly those who are severely obese, experience psychological disorders. Rates of mental disorders tend to be higher among those with obesity than they are in the general population.424-428
Depression
Individuals with severe obesity are approximately five times more likely to have experienced a significant depressive episode in the previous year than those who have an average weight.429 The association between depression and obesity appears higher in women than in men.430 A study revealed a higher prevalence of depressive symptoms among obese adolescents compared to the normal weight group.431 Similarly, in another study, clinically obese adolescents exhibited higher depression ratings than non-clinically obese and normal-weight adolescents.432 Obese children and adolescents often face difficulties in forming friendships as their peers perceive them as physically unattractive, less social, and more aggressive.433 Additionally, bullying from peers and family members contributes to the increased incidence of depression in overweight children.434-435
It has been demonstrated that females who perceive themselves as overweight,436 individuals aged ≥ 15 with obesity,437 children with obesity,438-439 and Class I and III obese females 440 have higher rates of suicidal ideations and attempts, which are potential consequences of depression. Nearly one-third of participants undergoing bariatric surgery exhibit clinically severe depressive symptoms at the time of surgery, with about half reporting a lifetime history of depression.441-442 Stigma and discrimination play significant roles in these findings. Negative weight-based assumptions, such as laziness, lack of willpower, low intelligence, and poor self-discipline, contribute to discrimination, prejudice, and stigma against individuals with obesity across different spheres of life.443-445
The relationship between obesity and depression is controversial and complex. While some articles concluded that there is no significant relationship between obesity and depression,446 Friedman suggested that the uncertainty in the results reflects the diverse experiences individuals have with obesity’s impacts. Some may face severe psychosocial issues, while others may experience minor problems, and some may be unaffected by it.423
Anxiety
Anxiety problems are prevalent among patients undergoing bariatric surgery, although their frequency is less clear in individuals receiving nonsurgical care. Social anxiety disorder, which affects around 9% of patients, is the most common anxiety issue among those seeking bariatric surgery.424 Individuals with severe obesity often report increased anxiety in social situations, which is unsurprising given Western society’s emphasis on leanness as a sign of physical attractiveness.447
Eating Disorders
Binge eating disorder is the most prevalent eating problem among obese people.448 A person with binge-eating disorder suffers a lack of control by eating too much food in a short period of time (less than two hours).448 Consequently, even when not hungry, the person eats considerably more quickly than usual and often eats alone. After eating, the person often expresses distaste.448 Studies have shown that obese adolescents,449 females,450 and youngsters451-452 are more predisposed to eating disorders. In addition, obesity in childhood seems to be associated with the development of eating problems in adulthood.453 Studies in the 1990s indicated that up to 50% of individuals presenting for bariatric surgery and a significant proportion of obese people seeking weight reduction therapy had binge-eating disorder.454-457 More recent research suggests that 5%–15% of applicants for bariatric surgery may have the disease.458
Night-eating syndrome is the second most prevalent eating disorder, affecting approximately 5% of individuals with obesity.459 Night-eating syndrome is characterized by disruptions in the circadian rhythm of food intake, leading individuals to wake during the night to eat.459,460
Bipolar Disorder
A recent study,461 which involved reviewing the four available studies in this field,462-465 has reported that people with obesity are more susceptible to developing bipolar disorder (pooled adjusted odds ratio: 1.32, 95% confidence interval: 1.01–1.62). Several studies have confirmed that bipolar disorder is a risk factor for obesity, but few studies have explored the impact of obesity on the development of bipolar disorder.
Prevention and Management of Obesity
Lifestyle Interventions
Due to the absence of specific pharmaceutical therapies for treating obesity, “lifestyle modification” continues to be the most common form of treatment.466 It is advised that individuals with obesity lose at least 10% of their body weight through lifestyle modifications (e.g., changes in diet, physical activity, and behavioral therapy).467 Implementing a portion-controlled diet can also result in significant short-term weight loss.468 A variety of nutritional approaches and strategies are available that can be tailored to meet the needs of individual patients.
Recent trends indicate a rise in the popularity of very low-calorie, Mediterranean, low-glycemic index, and low-carbohydrate diets.469 Effective weight management can be achieved through regular physical activity, with aerobic exercise being recommended for all patients with obesity. It is recommended that patients engage in either 75 minutes of vigorous-intensity aerobic activity per week or 150 minutes of moderate-intensity exercise per week.470 Exercise has been shown to improve metabolic endpoints, reduce weight, and prevent weight gain.470 Additionally, anaerobic exercise, also known as resistance or strength training, is often more effective in reducing body fat percentage while increasing muscle mass.471 Leisure time and occupational activities can also contribute to a patient’s physical activity regimen.472,473 For patients with obesity, behavioral changes should be tailored to their individual needs. It is crucial to conduct a thorough evaluation of the patient’s current and routine behaviors before offering personalized recommendations aimed at addressing those behaviors or barriers.474 In many cases, the adoption of lifestyle modifications yields a substantial decrease in body mass.474 Furthermore, previous research suggests that effective holistic approaches that consider cultural norms, environmental practices, home activities, and supportive educators and parents are required for managing and preventing obesity in children.475,476
Given that individuals’ food choices are largely influenced by their environment, it is crucial for governments to enhance their policies and environment to reduce the prevalence of unhealthy food options and increase the accessibility of healthy alternatives.477 Restrictions should be placed on the availability of obesogenic foods marketed to children, and policies should be tailored to incentivize the production of foods containing lower levels of salt, sugar, and fat.478 A small tax has been imposed on unhealthy foods in several regions of the US and Canada to limit consumption.479 It is imperative to raise awareness among policymakers and practitioners regarding the potential influence of food advertising on human health and behavior. Further, it is recommended that food manufacturers be incentivized to create and distribute weight-friendly food products. Nutrition educators should teach the correct way to implement food advertising.480 Several European nations, including Denmark, Norway, Sweden, and Austria, have restrictions on television advertising to young children.481 Interventions aimed at encouraging behavioral modifications (e.g., social marketing, nutrition education, health promotion, and incentives for healthy living), alongside measures that address the causes of obesity (e.g., policy changes, laws, and regulations), are expected to yield significant results in reducing the growing problem of obesity.482
Pharmacotherapy
Over the past six decades, the treatment of obesity through medication has advanced considerably. Currently, the Federal Drug Administration (FDA) has approved seven anti-obesity medications for long-term use.483 These drugs are recommended in conjunction with lifestyle changes, similar to treatments for other chronic diseases. Current guidelines recommend weight loss medications for individuals with a BMI of ≥ 30 kg/m2 or those with a BMI of ≥ 27 kg/m2 who also have an obesity-related health issue, especially if they have already tried lifestyle improvements without success.484 Currently, setmelanotide is prescribed for specific rare obesity syndromes, including Bardet-Biedl syndrome.483,485 In addition, six other drugs (orlistat, phentermine/topiramate, naltrexone/bupropion, liraglutide, semaglutide, and tirzepatide) have been approved for treating general obesity.483 Most of these drugs function centrally to reduce appetite, increase fullness, and act on the digestive system to slow stomach emptying.486
Orlistat
Orlistat, as a semisynthetic derivative of lipstatin, is a potent inhibitor of GI lipase, leading to reduced fat absorption.487 It is indicated for individuals with a BMI of 30 kg/m2 or more, or 28 kg/m2 if other obesity-related health issues are present.488 Clinical studies demonstrate that when combined with a modified lifestyle and low-fat diet, orlistat effectively aids weight loss and alleviates obesity-related conditions more effectively than diet alone. However, over 20% of users experienced side effects related to fat malabsorption in the first year, such as oily stools and abdominal pain. While these side effects decreased in frequency during the second year, the long-term efficacy and side effect profile of the drug remain unknown beyond two years.489
Phentermine/Topiramate
In 2012, the U.S. Food and Drug Administration approved the combined use of phentermine and topiramate to treat obesity.490 Aronne et al conducted a clinical trial to compare the combined use of phentermine and extended-release topiramate with individual doses of each drug and a placebo in adults with obesity. The combination treatment proved more effective in promoting weight loss and resulted in greater weight loss compared to both the placebo and individual drug treatments. Specifically, the combined doses of 7.5/46 mg and 15/92 mg led to weight losses of 8.5% and 9.2%, respectively, and a higher proportion of subjects on the combination treatment achieved a weight loss of 5% or more when compared with those on placebo or the monotherapies.491
Smith et al, performing a systematic review to assess the pharmacology, efficacy, and safety of the phentermine/topiramate combination for managing obesity, concluded that PHEN/TPM showed statistically significant weight loss compared to the placebo, with the most notable reduction being 10.6% after 56 weeks with the 15/92 mg dose. This weight loss persisted over two years, with a significant proportion of patients achieving weight reductions of over 5%, 10%, or 15%. The drug also reduced waist circumference, fasting triglycerides, and fasting glucose. The common side effects included paresthesia, dizziness, insomnia, and dry mouth. In conclusion, while PHEN/TPM is effective for weight loss and has received approval as an adjunct treatment, long-term safety data are still necessary.492
Another meta-analysis by Lei et al revealed that phentermine/topiramate therapy led to a significant average weight loss of 7.73 kg compared to a placebo, with the extent of weight loss being dose-dependent. Different doses resulted in varied levels of weight loss, with the 15/92 mg dose being the most effective. Participants taking phentermine/topiramate achieved weight reductions based on their individual baseline weights. However, this therapy was also associated with several side effects, mainly related to the nervous system, such as dysgeusia, paresthesia, and dry mouth. In addition to weight loss, the drug also positively affected waist circumference, blood pressure, blood sugar, and lipid levels.493
Naltrexone/Bupropion
Naltrexone and bupropion are both individually FDA-approved for different purposes. Bupropion, a dopamine/norepinephrine reuptake inhibitor, is approved for treating depression and aiding smoking cessation. Conversely, naltrexone, an opioid receptor antagonist, is approved for managing alcohol and opioid addiction.484 However, in September 2014, FDA approval was received for the use of these drugs in combination to treat obesity.484 The recommended dosage of the combination typically starts low and increases weekly until two tablets taken twice daily, providing 360 mg of bupropion and 32 mg of naltrexone. Four large studies (Contrave obesity research trials) involving over 4500 participants (4536 patients) demonstrated significant weight loss benefits with the combination therapy when compared to a placebo. In one trial (COR-I), the average weight loss with the drugs was 6.1% compared to 1.3% with the placebo. The studies also noted improvements in cardiovascular risk factors, such as waist circumference, high-density lipoprotein cholesterol (HDL-C), and triglyceride levels. Additionally, diabetic participants in a COR-diabetes trial experienced a 0.6% reduction in hemoglobin A1c. However, similar to any medication, this treatment has been found to have a number of potential side effects, including nausea, dizziness, and dry mouth. It is also important to note that there may be risks when it is used with other medications or in individuals with specific medical conditions.495-497
Liraglutide
Liraglutide, a long-acting human glucagon-like peptide-1 (GLP-1) analog, offers therapeutic benefits for individuals with metabolic conditions such as T2DM and obesity.498 This GLP-1 analog helps decrease food consumption, facilitate weight loss, and enhance metabolic functions. Its primary impact on appetite, metabolism, and weight reduction stems from its influence on both peripheral and central pathways, specifically activating the hindbrain and hypothalamus.499
A 56-week randomized controlled trial (RCT) involving 3,731 non-diabetic patients with obesity or overweight and associated health issues investigated the potential of liraglutide’s weight management potential. Administered as a daily 3.0 mg subcutaneous injection, liraglutide demonstrated significant weight loss compared to a placebo. Patients treated with liraglutide experienced an average weight loss of 8.4 kg, which was significantly higher than the 2.8 kg lost by the placebo group. In addition, 63.2% of the participants on liraglutide lost at least 5% of their body weight, with 33.1% losing over 10%, compared to 27.1% and 10.6%, respectively, in the placebo group. The common side effects included mild nausea and diarrhea. The study, published in the New England Journal of Medicine, concluded that liraglutide, combined with diet and exercise, aids in weight reduction and metabolic control.500 Furthermore, a meta-analysis of seven RCTs involving 6028 adults with obesity or overweight for at least one year indicated that liraglutide 3.0 mg significantly reduced weight compared to a placebo. However, it also revealed a higher incidence of adverse events among liraglutide users, resulting in more treatment discontinuations. Notably, liraglutide had a more pronounced effect on weight reduction in participants without DM.501
Setmelanotide
Setmelanotide is a drug that targets the MC4R and is used to treat extreme obesity due to genetic conditions, such as POMC, PCSK1, or LEPR deficiencies.502,503 The FDA approved setmelanotide on November 25, 2020, for chronic weight management in individuals aged ≥ 6 with deficiencies in POMC, PCSK1, or LEPR.504 Setmelanotide represents a personalized approach to obesity treatment. Semaglutide 2.4 mg provides twice the weight loss compared to older drugs used to treat obesity and overweight conditions with comorbidities. This medication has already been used for diabetes as a GLP-1 receptor analog.505
In a multicenter phase 3 trial that was conducted across seven countries, participants with POMC or LEPR deficiency obesity were given setmelanotide. The main goal was to obtain a 10% weight loss from baseline within 12 months, with 80% of POMC trial participants and 45% of LEPR trial participants meeting this goal. Significant reductions in hunger scores were also noted. However, common side effects such as injection site reactions and hyperpigmentation were found as well. The findings of this revealed that setmelanotide could effectively treat obesity stemming from POMC or LEPR deficiencies.506 In another study performed by Collet et al, setmelanotide induced weight loss in obese individuals with MC4R variants during a 28-day clinical trial, particularly among those with POMC defects. The drug was also found to be effective for individuals with MC4R deficiency.507
Semaglutide
A meta-analysis of 12 clinical trials revealed that semaglutide is both safe and effective for treating obesity, although some users reported GI side effects. Further research with larger samples and longer follow-up periods is needed to understand its long-term effects, optimal dosages, and appropriate treatment durations for a wider range of individuals with overweight or obesity.508
In a Phase 3 trial involving adults with obesity or overweight but without T2DM, the oral glucagon-like peptide-1 analog, semaglutide 50 mg taken once daily, was compared to a placebo to assess its effectiveness and safety. The findings of this trial, which was conducted in 50 outpatient clinics across Asia, Europe, and North America, confirmed that participants on semaglutide experienced an average weight loss of 15.1% over 68 weeks compared to 2.4% in the placebo group. Furthermore, 85% of participants on semaglutide achieved a body weight reduction of at least 5% in comparison to only 26% on the placebo. In conclusion, oral semaglutide 50 mg demonstrated significant weight loss benefits compared to a placebo in adults with obesity or overweight without T2DM.509
Another study by Ghusn et al investigated the effects of weekly semaglutide injections (1.7 mg or 2.4 mg) for 3–6 months in patients with obesity or overweight. On average, they experienced a weight loss of 6.7 kg (5.9%) after 3 months and 12.3 kg (10.9%) after 6 months. Interestingly, 87.3% of these patients shed over 5% of their weight by the 6-month mark. However, those with T2DM experienced less weight loss than those without T2DM. The results related to semaglutide’s effectiveness in weight loss conform to the findings of a randomized clinical trial.510 The STEP 5 trial evaluated the effectiveness of semaglutide 2.4 mg injections for weight loss in adults over 104 weeks. Compared to the placebo group (2.6%), those on semaglutide experienced an average weight reduction of 15.2%. However, more GI side effects were reported in the semaglutide group. Overall, semaglutide was significantly more effective than the placebo for weight loss over two years.511
Tirzepatide
Tirzepatide is a novel GLP-1 and gastric inhibitory polypeptide receptor agonist administered by once-weekly subcutaneous injection.512 It is effective in the treatment of obesity in patients with and without DM.513
In an open-label trial including over 1,800 patients with diabetes, once-weekly tirzepatide, in varying doses, was compared with semaglutide 1 mg.514 After 40 weeks, reduction in body weight with all doses of tirzepatide was greater compared with semaglutide (5 mg, 10 mg, and 15 mg of tirzepatide; -7.6 kg, -9.3 kg, and -11.2 kg, respectively: 1 mg of semaglutide; -5.7 kg).514
In addition, in a double-blind placebo-controlled randomized trial including over 2500 adults with obesity (but without diabetes), tirzepatide once weekly was compared with the placebo.513 After 72 weeks, reduction in body weight at all tirzepatide doses (5 mg, 10 mg, and 15 mg) was more noticeable in comparison with the placebo (-16.1 kg, -22.2 kg, and -23.6, respectively, versus -2.4 kg).513
Gelesis100
Gelesis100 is a non-systemic hydrogel composed of modified cellulose combined with citric acid, designed for the treatment of overweight or obesity. When taken orally with water before meals, it rapidly absorbs water in the stomach, evenly mixing with food. The hydrated Gelesis100 occupies approximately one-fourth of the average stomach volume, forming thousands of small gel-like pieces with a consistency similar to solid foods such as vegetables yet containing no calories. While it retains its form in the small intestine, it starts to break down in the large intestine, releasing the absorbed water and leaving behind waste material.515 Classified as a medical device, Gelesis100 operates mechanically and has received a non-significant risk designation from the FDA.516
A 24-week randomized, double‐blind, placebo‐controlled study evaluated the effectiveness and safety of Gelesis100 for treating overweight or obesity. The study findings demonstrated that Gelesis100 led to a significantly greater weight loss (6.4%) than the placebo (4.4%). Notably, 59% of Gelesis100-treated individuals achieved at least 5% weight loss, and 27% lost over 10%, compared to 42% and 15% in the placebo group, respectively. Moreover, participants on Gelesis100 had twice the odds of achieving 5% or 10% weight loss, and prediabetic or drug-naive type 2 diabetic patients were six times more likely to achieve over 10% weight loss. Gelesis100 displayed no additional safety risks and is considered a promising treatment option for overweight and obesity issues.515 However, despite the FDA approval, the American Gastroenterological Association guidelines currently list the use of Gelesis100 as a knowledge gap, highlighting the need for more research and evidence.
Bariatric Surgery
Several studies have confirmed the potential and efficacy of bariatric surgery as a therapeutic approach for treating obesity and obesity-related morbidities. These studies have explored various methods, all of which aimed to interfere with the physiological processes of eating, digestion, and absorption.517 Sleeve gastrectomy (SG) and RYGB have emerged as the two most commonly performed procedures, accounting for 61% and 17% of primary bariatric surgeries, respectively, with RYGB considered the gold standard.518 However, techniques such as jejunoileal bypass, vertical banded gastroplasty, and laparoscopic adjustable gastric banding (LAGB) have been largely abandoned due to concerns regarding side effects, frequent reoperation needs, or limited long-term effectiveness.519
The National Health System and other guidelines have established indicators for bariatric surgery, including a BMI ≥ 40 kg/m2 or a BMI ≥ 35 kg/m2 in patients with obesity-related morbidities. Furthermore, patients with poorly controlled T2DM and a BMI of 30–35, despite optimal pharmacological therapy, may also be candidates.520,521 Multiple studies have consistently demonstrated the superior efficacy of bariatric surgery in achieving short- and long-term DM remission, particularly in earlier-stage patients before requiring multiple medications.522,523
The findings of RCTs comparing different bariatric procedures have been controversial. Some studies have found that SG and RYGB outperform each other in terms of weight loss outcomes, while other studies have reported similar levels of efficacy.524 A systematic review and meta-analysis of six RCTs revealed no significant difference in BMI reduction between the two methods.525 Another systematic review and meta-analysis, which included 20 RCTs comparing SG and RYGB, concluded that both methods achieved similar BMI reductions 1 month, 3 months, 2 years, and 5 years after surgery while favoring RYGB after 1 year. Additionally, excess weight loss at 3 months, 6 months, 12 months, and 24 months after surgery showed no significant differences between the methods; however, RYGB outscored SG by 11.93 and 13.11 percentage units after 3 years and 5 years, respectively.526 Furthermore, another systematic review and meta-analysis, encompassing both RCTs and observational studies comparing SG and RYGB, indicated that RYGB had greater efficacy in total weight loss and the remission of T2DM, dyslipidemia, and HTN at five-year follow-ups.527
Beyond weight loss, bariatric surgery has shown substantial efficacy in managing obesity-related morbidities, including T2DM, dyslipidemia, HTN, sleep apnea, osteoarthritis, urinary incontinence, and cancer.519 Multiple RCTs on obese patients with T2DM revealed that various bariatric surgeries were more effective than non-surgical therapies for glycemic control and remission of T2DM, with varying rates of remission at different time points ranging from 1 to 5 years post-surgery.528-532 A systematic review and meta-analysis of these RCTs represented that single anastomosis (mini) gastric bypass (mini-GBP), SG, RYGB, LAGB, and a biliopancreatic diversion (BPD) without duodenal switch (DS) can all yield significantly more favorable outcomes in terms of T2DM remission and glycemic control in comparison to non-surgical interventions.533 Among these methods, mini-GBP, which includes both simplicity and reversibility, had the highest efficacy.534 Similarly, the significant preventive effects of bariatric surgeries and their superiority over medical treatments for both microvascular and macrovascular complications of T2DM have been well-established.535-537
Furthermore, several RCTs have evaluated the efficacy of bariatric surgery in treating dyslipidemia in patients with obesity. The findings of the largest RCT, involving 134 obese patients and comparing the effects of different bariatric surgeries with intensive medical therapy in terms of dyslipidemia resolution, confirmed that RYGB and SG reduced triglyceride levels by 40% and 29%, respectively, over 5 years from baseline, compared to 8% with intensive medical therapy.532 Likewise, RYGB, SG, and intensive medical therapy enhanced HDL-C concentration by 32%, 30%, and 7%, respectively, demonstrating the superior performance of bariatric surgeries over medical therapies in ameliorating dysregulated lipid profiles in patients with obesity. An analysis of findings from 35 RCTs demonstrated that mini-GBP, RYGB with omentectomy, and DS had the best performance in reducing triglycerides, cholesterol, and LDL-C levels. On the other hand, SG with omentectomy was found to be most effective in elevating HDL-C concentrations.538
In terms of HTN, different findings have been made, depending on the length of the follow-up period and the type of surgery performed, with the majority suggesting promising outcomes. A meta-analysis of studies with a mean follow-up of 34 months indicated that bariatric surgery has been associated with a 68% decrease in obesity-related HTN.539 Likewise, another meta-analysis reported that HTN was resolved in 38.4% of patients who received LAGB, 75.4% of patients who underwent RYGB, 72.5% of patients who received vertical banded gastroplasty, and 81.3% of patients who had BPD-DS.540 Nevertheless, the long-term effects of these interventions on HTN are not known yet. A study suggested that within 10 years of follow-up, approximately 44% of patients who had previously achieved the resolution of HTN experienced its recurrence and required anti-hypertensive medication for blood pressure control.541
Public Health Policies and Interventions
Previous research highlighted obesity as a global concern, emphasizing that its control requires efforts beyond personal prevention.542 Nutrition policies play a crucial role in enhancing the quality of diets and reducing the growing prevalence of obesity. Various measures, such as beverage taxes and recent nutritional modifications for women, infants, and children in programs such as the National School Lunch Program and School Breakfast Program, have been identified to be effective in promoting healthier consumption.543 The implementation of calorie labeling on prepared foods has been associated with modest decreases in the calories purchased from such foods. Cost-effectiveness analyses suggest that beverage taxes, calorie labeling, and adjustments to federal nutrition assistance programs are expected to be economically beneficial in curbing the rise in obesity prevalence. Additionally, it is noted that a combination of multiple policies is more likely to succeed than relying on single policies.543
Calorie Labeling
Addressing obesity requires a comprehensive and multifaceted approach. One aspect of this solution may involve providing calorie information on menus for individual dishes and drinks. While the existing evidence does not strongly support a substantial reduction in calories ordered, the implementation of menu calorie labeling serves as a cost-effective educational strategy. It has the potential to influence consumers to make slightly lower calorie purchases.544 The inclusion of calorie labels has the potential to assist consumers in making healthier choices. Furthermore, it motivates manufacturers to reformulate their products or offer a greater variety of lower-calorie options. While there is not extensive evidence indicating that menu labeling significantly impacts calorie purchases in fast-food establishments, some studies suggest that it does lead to a reduction in calories purchased in specific restaurant types and cafeteria environments. The limited available data on altered calorie labels indicate that they can promote the choice of lower-calorie options, although their effects may not significantly differ from those of standard calorie labels alone.544,545
Food Taxing
Taxation and increased strategies have shown promise in limiting calorie intake. For example, in studies involving university students, raising the prices of high-calorie foods resulted in a decrease in the proportion of selected calories. Therefore, implementing a tax on high-calorie foods has the potential to reduce calorie demand, making it a viable policy measure to combat the prevalence of obesity.546 However, it is important to note that while small taxes could generate significant revenue, they may not significantly affect obesity rates. Conversely, high excise taxes could directly influence weight in at-risk populations but might face challenges in terms of political acceptance and sustainability.547 A frequently cited objection to taxing unhealthy foods is the concern about infringing upon individual freedom. Nevertheless, it should be considered that the broader society bears the costs of obesity resulting from poor nutritional choices, including diminished productivity and costs to the healthcare system.547
A comprehensive systematic review and meta-analysis study assessed the impact of SSB taxes on the prevalence of overweight and obesity across countries with different income levels. Among the sixteen studies analyzed, most indicated a decrease in consumption, sales, and purchases with increasing SSB prices. Eight of the studies suggested the positive effect of SSB taxes on reducing the prevalence of overweight and obesity. A 20% tax was estimated to be more effective than a 10% rate in decreasing these health issues. Conversely, studies from high-income countries showed no significant effects, while those from upper-middle- and middle-income countries demonstrated notable reductions in purchase, consumption, and obesity prevalence. These findings indicate that a high SSB tax, especially if specific to beverage volume, could serve as an effective fiscal policy measure to lower SSB consumption and combat overweight and obesity.548
In a comprehensive study conducted in Kenya, it was revealed that implementing a 20% tax on SSBs, mandatory kilojoule menu labeling, changes in consumption patterns related to supermarket food purchases, and a shift in national consumption back to 1975 energy intake levels had significant impacts on health-adjusted life years (HALYs). The estimated effects ranged from 151,718 to 13.1 million HALYs, equating to 3 to 261 HALYs per 1,000 persons. Additionally, it was estimated to save between USD 0.08 billion and USD 6.2 billion, with productivity gains ranging from USD 1.2 billion to USD 92 billion. Both the 20% SSB tax and menu intake energy labeling were deemed cost-effective, promoting health and saving costs.549
Physical Activities Policies
Most children and adolescents do not engage in sufficient physical activity, which is considered a vital aspect of a healthy lifestyle. Public health initiatives must address this lack of physical activity among young people by implementing interventions such as school programs, educational initiatives, and after-school activities. It is crucial to ensure equitable access to community resources, promote youth sports, re-establish active transportation to school, and create environments conducive to physical activity in homes. Comprehensive national and international strategic planning is necessary to effectively implement these initiatives and promote a culture of physical activity among children and adolescents.550
Limitations
The present review article relies on existing published studies, which may have inherent limitations such as sampling bias, measurement errors, or variations in methodologies across studies. The accuracy and reliability of the data used in this review depend on the quality of the sources. It is important to note that this study employed a narrative review rather than a systematic review and meta-analysis, making it harder to draw definitive conclusions. Given that obesity is a dynamic and evolving field, new research and developments may have emerged since our search was conducted. Therefore, this review may not capture the most up-to-date information and trends related to obesity. The review process may be biased, as the authors’ perspectives and judgments may influence the selection of studies, data extraction, and synthesis of findings. Additionally, the quality and rigor of the included studies may vary, potentially affecting the overall validity and reliability of the conclusions drawn.
The findings presented in this review may not be universally applicable as they are based on studies conducted in specific populations or regions. The epidemiology, risk factors, and trends of obesity can vary across different geographic locations, socioeconomic groups, and cultural contexts. Hence, caution should be exercised when generalizing the results to other populations. Moreover, we focused solely on specific risk factors, although other potential risk factors may also warrant exploration. Additionally, some topics covered under risk factors may have needed a more balanced approach. While this review explored various risk factors associated with obesity, establishing causal relationships can be challenging due to the presence of confounding factors, unmeasured variables, or reverse causality in the original studies, which may limit the ability to attribute the observed associations to the discussed risk factors.
It is essential to consider these limitations when interpreting the findings of this study and to encourage further research to address these gaps and refine our understanding of the epidemiology and risk factors of obesity. Despite these limitations, our comprehensive review identified crucial gaps in our understanding and highlighted areas that require further investigation. Future research should prioritize conducting systematic reviews, with or without meta-analysis, on specific risk factors, especially those with inconsistent findings, to clarify whether there is a correlation.
Conclusion
In general, obesity remains a major public health concern worldwide. This review article highlighted its prevalence, risk factors, and trends, demonstrating its persistent rise and resulting in considerable health and economic burdens. Genetic, environmental, and behavioral factors contribute to the development of obesity. Understanding these risk factors is vital for effective prevention and management strategies. Addressing the obesity epidemic requires a comprehensive approach involving individuals, communities, healthcare systems, and policymakers. In addition, implementing evidence-based interventions, such as promoting healthy eating, regular physical activity, and supportive environments, is essential to combat this global health challenge. Continued research and surveillance play a crucial role in monitoring trends, identifying emerging risk factors, and evaluating the effectiveness of interventions in reducing the prevalence of obesity. By prioritizing prevention and implementing multidisciplinary strategies, we can work toward a healthier future, reducing the burden of obesity and its associated complications.
Acknowledgments
We would like to express our sincere gratitude to the Social Determinants of Health Research Center at Shahid Beheshti University of Medical Sciences for their invaluable support and contribution to this research. Additionally, we acknowledge the Global Burden of Disease Study, led by the Institute for Health Metrics and Evaluation, for providing publicly available data on global epidemiology, which was crucial to the development of this work.
Author contributions
Conceptualization: Saeid Safiri and Ali-Asghar Kolahi.
Data curation: Seyed Aria Nejadghaderi6 and Asra Fazlollahi.
Formal analysis: Saeid Safiri.
Funding acquisition: Ali-Asghar Kolahi.
Investigation: Saeid Safiri and Ali-Asghar Kolahi.
Methodology: Saeid Safiri and Ali-Asghar Kolahi.
Resources: Ali-Asghar Kolahi
Software: Saeid Safiri.
Supervision: Saeid Safiri and Ali-Asghar Kolahi.
Validation: Saeid Safiri and Ali-Asghar Kolahi.
Visualization: Saeid Safiri.
Writing–original draft: Saeid Safiri, Amin Daei Sorkhabi, Reza Aletaha, Sana Hamidi, Kimia Motlagh Asghari, Aila Sarkesh, Sina Janbaz Alamdary, Amir Ghaffari Jolfayi, Seyed Aria Nejadghaderi, Asra Fazlollahi, Reza Mohammadinasab, Mark J. M. Sullman, Nahid Karamzad, Fikrettin Sahin, and Ali-Asghar Kolahi.
Writing–review & editing: Reza Aletaha, Amir Ghaffari Jolfayi, Asra Fazlollahi, Mark J. M. Sullman, Fikrettin Sahin, and Ali-Asghar Kolahi.
Data availability statement
Not applicable.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no competing interests.
References
- Antipatis VJ, Gill TP. Obesity as a global problem. In: Björntorp P, ed. International Textbook of Obesity. Wiley; 2001. p. 1-22.
- World Health Organization. 2020 Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1002/0470846739.
- Yanovski JA. Obesity: trends in underweight and obesity - scale of the problem. Nat Rev Endocrinol 2018; 14(1):5-6. doi: 10.1038/nrendo.2017.157 [Crossref] [ Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 390(10113):2627-42. doi: 10.1016/s0140-6736(17)32129-3 [Crossref] [ Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387(10026):1377-96. doi: 10.1016/s0140-6736(16)30054-x [Crossref] [ Google Scholar]
- Pourali F, Araj-Khodaei M, Sahebihagh MH, Taheri-Targhi S, Karamzad N, Villani A. Obesity prevalence among older people in Tabriz, Iran: data from health status of aged people in Tabriz (HSA-T) study. Int J Aging 2023; 1(1):e8. doi: 10.34172/ija.2023.e8 [Crossref] [ Google Scholar]
- Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process A position statement of the World Obesity Federation. Obes Rev 2017; 18(7):715-23. doi: 10.1111/obr.12551 [Crossref] [ Google Scholar]
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019; 92:6-10. doi: 10.1016/j.metabol.2018.09.005 [Crossref] [ Google Scholar]
- Lam BC, Lim AY, Chan SL, Yum MP, Koh NS, Finkelstein EA. The impact of obesity: a narrative review. Singapore Med J 2023; 64(3):163-71. doi: 10.4103/singaporemedj.SMJ-2022-232 [Crossref] [ Google Scholar]
- Sharma AM, Padwal R. Obesity is a sign - over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev 2010; 11(5):362-70. doi: 10.1111/j.1467-789X.2009.00689.x [Crossref] [ Google Scholar]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019; 15(5):288-98. doi: 10.1038/s41574-019-0176-8 [Crossref] [ Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378(9793):804-14. doi: 10.1016/s0140-6736(11)60813-1 [Crossref] [ Google Scholar]
- Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am 2018; 102(1):13-33. doi: 10.1016/j.mcna.2017.08.004 [Crossref] [ Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013; 9(1):13-27. doi: 10.1038/nrendo.2012.199 [Crossref] [ Google Scholar]
- Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries. The National Academies Collection: Reports funded by National Institutes of Health. In: Fuster V, Kelly BB, eds. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health. Washington, DC: National Academies Press (U); 2010. doi: 10.17226/12815.
- Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012; 13(8):659-80. doi: 10.1111/j.1467-789X.2011.00982.x [Crossref] [ Google Scholar]
-
Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med 2014;127(8):717-27.e12. doi: 10.1016/j.amjmed.2014.02.026.
- Mousavi SE, Fazlollahi A, Nejadghaderi SA, Aslani A, Sullman MJ, Kolahi AA. The burden of ischemic heart disease among adults 70 years and older in Iran, 1990-2019. Int J Aging 2023; 1(1):e9. doi: 10.34172/ija.2023.e9 [Crossref] [ Google Scholar]
- Lavie CJ, Arena R, Alpert MA, Milani RV, Ventura HO. Management of cardiovascular diseases in patients with obesity. Nat Rev Cardiol 2018; 15(1):45-56. doi: 10.1038/nrcardio.2017.108 [Crossref] [ Google Scholar]
- Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved?. Integr Pharm Res Pract 2019; 8:1-11. doi: 10.2147/iprp.S133088 [Crossref] [ Google Scholar]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81(9):646-56. [ Google Scholar]
- Noori M, Mousavi SE, Motlagh Asghari K, Nejadghaderi SA, Sullman MJ, Kolahi AA. The burden of cancers and their attributable risk factors among Iranian adults aged 70 and above, 1990-2019. Int J Aging 2023; 1(1):e5. doi: 10.34172/ija.2023.e5 [Crossref] [ Google Scholar]
- Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev 2001; 2(4):219-29. doi: 10.1046/j.1467-789x.2001.00040.x [Crossref] [ Google Scholar]
- Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the US. Diabetes Care 2009; 32(12):2225-9. doi: 10.2337/dc09-0459 [Crossref] [ Google Scholar]
- Bray GA. History of obesity. In: Williams G, Frühbeck G, eds. Obesity: Science to Practice. Oxford, UK: Wiley‐Blackwell; 2009. p. 3-18.
- Spitzer M. A further look at obesity. Lancet 2010; 376(9747):1144; author reply-5. doi: 10.1016/s0140-6736(10)61534-6 [Crossref] [ Google Scholar]
- Cheng TO. Hippocrates and cardiology. Am Heart J 2001; 141(2):173-83. doi: 10.1067/mhj.2001.112490 [Crossref] [ Google Scholar]
- Haslam D. Obesity: a medical history. Obes Rev 2007; 8 Suppl 1:31-6. doi: 10.1111/j.1467-789X.2007.00314.x [Crossref] [ Google Scholar]
- Papavramidou N, Christopoulou-Aletra H. Management of obesity in the writings of Soranus of Ephesus and Caelius Aurelianus. Obes Surg 2008; 18(6):763-5. doi: 10.1007/s11695-007-9362-1 [Crossref] [ Google Scholar]
- Papavramidou N, Christopoulou-Aletra H. Greco-Roman and Byzantine views on obesity. Obes Surg 2007; 17(1):112-6. doi: 10.1007/s11695-007-9017-2 [Crossref] [ Google Scholar]
- Papavramidou NS, Papavramidis ST, Christopoulou-Aletra H. Galen on obesity: etiology, effects, and treatment. World J Surg 2004; 28(6):631-5. doi: 10.1007/s00268-004-7458-5 [Crossref] [ Google Scholar]
- Cumston CG. An Introduction to the History of Medicine: From the Time of the Pharaohs to the End of the XVIIIth Century. London: Dawsons of Pall Mall; 1968.
- Nikaein F, Zargaran A, Mehdizadeh A. Rhazes’ concepts and manuscripts on nutrition in treatment and health care. Anc Sci Life 2012; 31(4):160-3. doi: 10.4103/0257-7941.107357 [Crossref] [ Google Scholar]
- Abdel-Halim RE. Obesity: 1000 years ago. Lancet 2005; 366(9481):204. doi: 10.1016/s0140-6736(05)66907-3 [Crossref] [ Google Scholar]
- Vari SG. Obesity: rubensian beauty turned into major health problem. Croat Med J 2017; 58(2):89-91. doi: 10.3325/cmj.2017.58.89 [Crossref] [ Google Scholar]
- Eknoyan G. A history of obesity, or how what was good became ugly and then bad. Adv Chronic Kidney Dis 2006; 13(4):421-7. doi: 10.1053/j.ackd.2006.07.002 [Crossref] [ Google Scholar]
- Short T. A Discourse Concerning the Causes and Effects of Corpulency: Together with the Method for Its Prevention and Cure. London: The Oxford Arms, Warwick Lane; 1728.
- Bray GA. A Guide to Obesity and the Metabolic Syndrome: Origins and Treatment. New York: CRC Press; 2011.
- Eknoyan G. Adolphe Quetelet (1796-1874)--the average man and indices of obesity. Nephrol Dial Transplant 2008; 23(1):47-51. doi: 10.1093/ndt/gfm517 [Crossref] [ Google Scholar]
- Nuttall FQ. Body mass index: obesity, BMI, and health: a critical review. Nutr Today 2015; 50(3):117-28. doi: 10.1097/nt.0000000000000092 [Crossref] [ Google Scholar]
- Agne AA, Daubert R, Munoz ML, Scarinci I, Cherrington AL. The cultural context of obesity: exploring perceptions of obesity and weight loss among Latina immigrants. J Immigr Minor Health 2012; 14(6):1063-70. doi: 10.1007/s10903-011-9557-3 [Crossref] [ Google Scholar]
- Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am 1967; 47(6):1345-51. doi: 10.1016/s0039-6109(16)38384-0 [Crossref] [ Google Scholar]
- Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res 2016; 118(11):1844-55. doi: 10.1161/circresaha.116.307591 [Crossref] [ Google Scholar]
- Soleimanpour H, Safari S, Sanaie S, Nazari M, Alavian SM. Anesthetic considerations in patients undergoing bariatric surgery: a review article. Anesth Pain Med 2017; 7(4):e57568. doi: 10.5812/aapm.57568 [Crossref] [ Google Scholar]
- Sumińska M, Podgórski R, Bogusz-Górna K, Skowrońska B, Mazur A, Fichna M. Historical and cultural aspects of obesity: from a symbol of wealth and prosperity to the epidemic of the 21st century. Obes Rev 2022; 23(6):e13440. doi: 10.1111/obr.13440 [Crossref] [ Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). GBD 2019 Cause and Risk Summary: High Body-Mass Index. Seattle, USA: IHME; 2020.
- Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: an analysis of the Global Burden of Disease Study. PLoS Med 2020; 17(7):e1003198. doi: 10.1371/journal.pmed.1003198 [Crossref] [ Google Scholar]
- Safiri S, Motlagh Asghari K, Sullman MJ. The global burden of diseases and injuries among older adults. Int J Aging 2023; 1:e16. doi: 10.34172/ija.2023.e16 [Crossref] [ Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008; 32(9):1431-7. doi: 10.1038/ijo.2008.102 [Crossref] [ Google Scholar]
- Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012; 70(1):3-21. doi: 10.1111/j.1753-4887.2011.00456.x [Crossref] [ Google Scholar]
- Alotaibi T, Almuhanna R, Alhassan J, Alqadhib E, Mortada E, Alwhaibi R. The relationship between technology use and physical activity among typically-developing children. Healthcare (Basel) 2020; 8(4):488. doi: 10.3390/healthcare8040488 [Crossref] [ Google Scholar]
- Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 2015; 17(3):327-35. doi: 10.31887/DCNS.2015.17.3/bbandelow [Crossref] [ Google Scholar]
- Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017. J Abnorm Psychol 2019; 128(3):185-99. doi: 10.1037/abn0000410 [Crossref] [ Google Scholar]
- Pellegrini M, Carletto S, Scumaci E, Ponzo V, Ostacoli L, Bo S. The use of self-help strategies in obesity treatment a narrative review focused on hypnosis and mindfulness. Curr Obes Rep 2021; 10(3):351-64. doi: 10.1007/s13679-021-00443-z [Crossref] [ Google Scholar]
- Singh S, Ricardo-Silgado ML, Bielinski SJ, Acosta A. Pharmacogenomics of medication-induced weight gain and antiobesity medications. Obesity (Silver Spring) 2021; 29(2):265-73. doi: 10.1002/oby.23068 [Crossref] [ Google Scholar]
- Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 2003; 37(3):193-220. doi: 10.1016/s0022-3956(03)00018-9 [Crossref] [ Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378(9793):804-14. doi: 10.1016/s0140-6736(11)60813-1 [Crossref] [ Google Scholar]
-
Committee on Accelerating Progress in Obesity Prevention; Food and Nutrition Board; Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. In: Glickman D, Parker L, Sim LJ, Del Valle Cook H, Miller EA, eds. Washington, DC: National Academies Press (US); 2012. doi: 10.17226/13275.
- Arkadianos I, Valdes AM, Marinos E, Florou A, Gill RD, Grimaldi KA. Improved weight management using genetic information to personalize a calorie controlled diet. Nutr J 2007; 6:29. doi: 10.1186/1475-2891-6-29 [Crossref] [ Google Scholar]
- Bort-Roig J, Gilson ND, Puig-Ribera A, Contreras RS, Trost SG. Measuring and influencing physical activity with smartphone technology: a systematic review. Sports Med 2014; 44(5):671-86. doi: 10.1007/s40279-014-0142-5 [Crossref] [ Google Scholar]
- Loos RJ, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet 2022; 23(2):120-33. doi: 10.1038/s41576-021-00414-z [Crossref] [ Google Scholar]
- Silventoinen K, Jelenkovic A, Sund R, Hur YM, Yokoyama Y, Honda C. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the COllaborative project of Development of Anthropometrical measures in Twins (CODATwins) study. Am J Clin Nutr 2016; 104(2):371-9. doi: 10.3945/ajcn.116.130252 [Crossref] [ Google Scholar]
- Luke A, Guo X, Adeyemo AA, Wilks R, Forrester T, Lowe W Jr. Heritability of obesity-related traits among Nigerians, Jamaicans and US black people. Int J Obes Relat Metab Disord 2001; 25(7):1034-41. doi: 10.1038/sj.ijo.0801650 [Crossref] [ Google Scholar]
- Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord 2004; 28(1):39-48. doi: 10.1038/sj.ijo.0802524 [Crossref] [ Google Scholar]
- Wu DM, Hong Y, Sun CA, Sung PK, Rao DC, Chu NF. Familial resemblance of adiposity-related parameters: results from a health check-up population in Taiwan. Eur J Epidemiol 2003; 18(3):221-6. doi: 10.1023/a:1023337917377 [Crossref] [ Google Scholar]
- Hunt SC, Hasstedt SJ, Kuida H, Stults BM, Hopkins PN, Williams RR. Genetic heritability and common environmental components of resting and stressed blood pressures, lipids, and body mass index in Utah pedigrees and twins. Am J Epidemiol 1989; 129(3):625-38. doi: 10.1093/oxfordjournals.aje.a115175 [Crossref] [ Google Scholar]
- Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet 2008; 9(11):819-30. doi: 10.1038/nrg2468 [Crossref] [ Google Scholar]
- Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol 2018; 6(3):223-36. doi: 10.1016/s2213-8587(17)30200-0 [Crossref] [ Google Scholar]
- Littleton SH, Berkowitz RI, Grant SFA. Genetic determinants of childhood obesity. Mol Diagn Ther 2020; 24(6):653-63. doi: 10.1007/s40291-020-00496-1 [Crossref] [ Google Scholar]
- Kaur Y, de Souza RJ, Gibson WT, Meyre D. A systematic review of genetic syndromes with obesity. Obes Rev 2017; 18(6):603-34. doi: 10.1111/obr.12531 [Crossref] [ Google Scholar]
- Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 2018; 27(20):3641-9. doi: 10.1093/hmg/ddy271 [Crossref] [ Google Scholar]
- Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 2016; 130(12):943-86. doi: 10.1042/cs20160136 [Crossref] [ Google Scholar]
- Wang DX, Kaur Y, Alyass A, Meyre D. A candidate-gene approach identifies novel associations between common variants in/near syndromic obesity genes and BMI in pediatric and adult European populations. Diabetes 2019; 68(4):724-32. doi: 10.2337/db18-0986 [Crossref] [ Google Scholar]
- Duis J, Butler MG. Syndromic and nonsyndromic obesity: underlying genetic causes in humans. Adv Biol (Weinh) 2022; 6(10):e2101154. doi: 10.1002/adbi.202101154 [Crossref] [ Google Scholar]
- Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev 2006; 27(7):710-18. doi: 10.1210/er.2006-0040 [Crossref] [ Google Scholar]
- Saeed S, Arslan M, Froguel P. Genetics of obesity in consanguineous populations: toward precision medicine and the discovery of novel obesity genes. Obesity (Silver Spring) 2018; 26(3):474-84. doi: 10.1002/oby.22064 [Crossref] [ Google Scholar]
- Saeed S, Bonnefond A, Manzoor J, Shabbir F, Ayesha H, Philippe J. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity (Silver Spring) 2015; 23(8):1687-95. doi: 10.1002/oby.21142 [Crossref] [ Google Scholar]
- Saeed S, Arslan M, Manzoor J, Din SM, Janjua QM, Ayesha H. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 2020; 69(7):1424-38. doi: 10.2337/db19-1238 [Crossref] [ Google Scholar]
- Farooqi IS. Genetic and hereditary aspects of childhood obesity. Best Pract Res Clin Endocrinol Metab 2005; 19(3):359-74. doi: 10.1016/j.beem.2005.04.004 [Crossref] [ Google Scholar]
-
Pigeyre M, Meyre D. Monogenic obesity. In: Freemark MS, ed. Pediatric Obesity: Etiology, Pathogenesis and Treatment. Cham: Springer; 2018. p. 135-52. doi: 10.1007/978-3-319-68192-4_8.
- Chung WK. An overview of mongenic and syndromic obesities in humans. Pediatr Blood Cancer 2012; 58(1):122-8. doi: 10.1002/pbc.23372 [Crossref] [ Google Scholar]
- Albuquerque D, Nóbrega C, Rodríguez-López R, Manco L. Association study of common polymorphisms in MSRA, TFAP2B, MC4R, NRXN3, PPARGC1A, TMEM18, SEC16B, HOXB5 and OLFM4 genes with obesity-related traits among Portuguese children. J Hum Genet 2014; 59(6):307-13. doi: 10.1038/jhg.2014.23 [Crossref] [ Google Scholar]
- Elkhenini HF, New JP, Syed AA. Five-year outcome of bariatric surgery in a patient with melanocortin-4 receptor mutation. Clin Obes 2014; 4(2):121-4. doi: 10.1111/cob.12051 [Crossref] [ Google Scholar]
- Baxter J, Armijo PR, Flores L, Krause C, Samreen S, Tanner T. Updates on monogenic obesity in a multifactorial disease. Obes Surg 2019; 29(12):4077-83. doi: 10.1007/s11695-019-04200-z [Crossref] [ Google Scholar]
- Koves IH, Roth C. Genetic and syndromic causes of obesity and its management. Indian J Pediatr 2018; 85(6):478-85. doi: 10.1007/s12098-017-2502-2 [Crossref] [ Google Scholar]
- Santoro N, del Giudice EM, Cirillo G, Raimondo P, Corsi I, Amato A. An insertional polymorphism of the proopiomelanocortin gene is associated with fasting insulin levels in childhood obesity. J Clin Endocrinol Metab 2004; 89(10):4846-9. doi: 10.1210/jc.2004-0333 [Crossref] [ Google Scholar]
- Potoczna N, Branson R, Kral JG, Piec G, Steffen R, Ricklin T. Gene variants and binge eating as predictors of comorbidity and outcome of treatment in severe obesity. J Gastrointest Surg 2004; 8(8):971-82. doi: 10.1016/j.gassur.2004.09.032 [Crossref] [ Google Scholar]
- Rohde K, Keller M, la Cour Poulsen L, Blüher M, Kovacs P, Böttcher Y. Genetics and epigenetics in obesity. Metabolism 2019; 92:37-50. doi: 10.1016/j.metabol.2018.10.007 [Crossref] [ Google Scholar]
- Su LN, Wang YB, Wnag CG, Wei HP. Network analysis identifies common genes associated with obesity in six obesity-related diseases. J Zhejiang Univ Sci B 2017; 18(8):727-32. doi: 10.1631/jzus.B1600454 [Crossref] [ Google Scholar]
- Choquet H, Kasberger J, Hamidovic A, Jorgenson E. Contribution of common PCSK1 genetic variants to obesity in 8,359 subjects from multi-ethnic American population. PLoS One 2013; 8(2):e57857. doi: 10.1371/journal.pone.0057857 [Crossref] [ Google Scholar]
- Rouskas K, Kouvatsi A, Paletas K, Papazoglou D, Tsapas A, Lobbens S. Common variants in FTO, MC4R, TMEM18, PRL, AIF1, and PCSK1 show evidence of association with adult obesity in the Greek population. Obesity (Silver Spring) 2012; 20(2):389-95. doi: 10.1038/oby.2011.177 [Crossref] [ Google Scholar]
- Mahmoud AM. An overview of epigenetics in obesity: the role of lifestyle and therapeutic interventions. Int J Mol Sci 2022; 23(3):1341. doi: 10.3390/ijms23031341 [Crossref] [ Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 2003; 33 Suppl:245-54. doi: 10.1038/ng1089 [Crossref] [ Google Scholar]
- Houshmand-Oeregaard A, Hansen NS, Hjort L, Kelstrup L, Broholm C, Mathiesen ER. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin Epigenetics 2017; 9:37. doi: 10.1186/s13148-017-0338-2 [Crossref] [ Google Scholar]
- Ott R, Stupin JH, Melchior K, Schellong K, Ziska T, Dudenhausen JW. Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin Epigenetics 2018; 10(1):131. doi: 10.1186/s13148-018-0567-z [Crossref] [ Google Scholar]
- Houde AA, Légaré C, Biron S, Lescelleur O, Biertho L, Marceau S. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med Genet 2015; 16:29. doi: 10.1186/s12881-015-0174-1 [Crossref] [ Google Scholar]
- Kim AY, Park YJ, Pan X, Shin KC, Kwak SH, Bassas AF. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun 2015; 6:7585. doi: 10.1038/ncomms8585 [Crossref] [ Google Scholar]
- Kühnen P, Handke D, Waterland RA, Hennig BJ, Silver M, Fulford AJ. Interindividual variation in DNA methylation at a putative POMC metastable epiallele is associated with obesity. Cell Metab 2016; 24(3):502-9. doi: 10.1016/j.cmet.2016.08.001 [Crossref] [ Google Scholar]
- Crujeiras AB, Campion J, Díaz-Lagares A, Milagro FI, Goyenechea E, Abete I. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept 2013; 186:1-6. doi: 10.1016/j.regpep.2013.06.012 [Crossref] [ Google Scholar]
- Perfilyev A, Dahlman I, Gillberg L, Rosqvist F, Iggman D, Volkov P. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am J Clin Nutr 2017; 105(4):991-1000. doi: 10.3945/ajcn.116.143164 [Crossref] [ Google Scholar]
- Stepanow S, Reichwald K, Huse K, Gausmann U, Nebel A, Rosenstiel P. Allele-specific, age-dependent and BMI-associated DNA methylation of human MCHR1. PLoS One 2011; 6(5):e17711. doi: 10.1371/journal.pone.0017711 [Crossref] [ Google Scholar]
- Drogan D, Boeing H, Janke J, Schmitt B, Zhou Y, Walter J. Regional distribution of body fat in relation to DNA methylation within the LPL, ADIPOQ and PPARγ promoters in subcutaneous adipose tissue. Nutr Diabetes 2015; 5(7):e168. doi: 10.1038/nutd.2015.19 [Crossref] [ Google Scholar]
- Love-Gregory L, Kraja AT, Allum F, Aslibekyan S, Hedman ÅK, Duan Y. Higher chylomicron remnants and LDL particle numbers associate with CD36 SNPs and DNA methylation sites that reduce CD36. J Lipid Res 2016; 57(12):2176-84. doi: 10.1194/jlr.P065250 [Crossref] [ Google Scholar]
- Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 2017; 541(7635):81-6. doi: 10.1038/nature20784 [Crossref] [ Google Scholar]
- Huang RC, Galati JC, Burrows S, Beilin LJ, Li X, Pennell CE. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin Epigenetics 2012; 4(1):21. doi: 10.1186/1868-7083-4-21 [Crossref] [ Google Scholar]
- Rohde K, Klös M, Hopp L, Liu X, Keller M, Stumvoll M. IRS1 DNA promoter methylation and expression in human adipose tissue are related to fat distribution and metabolic traits. Sci Rep 2017; 7(1):12369. doi: 10.1038/s41598-017-12393-5 [Crossref] [ Google Scholar]
- Milagro FI, Gómez-Abellán P, Campión J, Martínez JA, Ordovás JM, Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int 2012; 29(9):1180-94. doi: 10.3109/07420528.2012.719967 [Crossref] [ Google Scholar]
- Ramos-Lopez O, Samblas M, Milagro FI, Riezu-Boj JI, Crujeiras AB, Martinez JA. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol Int 2018; 35(7):969-81. doi: 10.1080/07420528.2018.1446021 [Crossref] [ Google Scholar]
- Pfeiffer S, Krüger J, Maierhofer A, Böttcher Y, Klöting N, El Hajj N. Hypoxia-inducible factor 3A gene expression and methylation in adipose tissue is related to adipose tissue dysfunction. Sci Rep 2016; 6:27969. doi: 10.1038/srep27969 [Crossref] [ Google Scholar]
- Su S, Zhu H, Xu X, Wang X, Dong Y, Kapuku G. DNA methylation of the LY86 gene is associated with obesity, insulin resistance, and inflammation. Twin Res Hum Genet 2014; 17(3):183-91. doi: 10.1017/thg.2014.22 [Crossref] [ Google Scholar]
- Hermsdorff HH, Mansego ML, Campión J, Milagro FI, Zulet MA, Martínez JA. TNF-alpha promoter methylation in peripheral white blood cells: relationship with circulating TNFα, truncal fat and n-6 PUFA intake in young women. Cytokine 2013; 64(1):265-71. doi: 10.1016/j.cyto.2013.05.028 [Crossref] [ Google Scholar]
- Gao W, Liu JL, Lu X, Yang Q. Epigenetic regulation of energy metabolism in obesity. J Mol Cell Biol 2021; 13(7):480-99. doi: 10.1093/jmcb/mjab043 [Crossref] [ Google Scholar]
- Castellano-Castillo D, Denechaud PD, Fajas L, Moreno-Indias I, Oliva-Olivera W, Tinahones F. Human adipose tissue H3K4me3 histone mark in adipogenic, lipid metabolism and inflammatory genes is positively associated with BMI and HOMA-IR. PLoS One 2019; 14(4):e0215083. doi: 10.1371/journal.pone.0215083 [Crossref] [ Google Scholar]
- Shanaki M, Omidifar A, Shabani P, Toolabi K. Association between HDACs and pro-inflammatory cytokine gene expressions in obesity. Arch Physiol Biochem 2022; 128(4):880-6. doi: 10.1080/13813455.2020.1734843 [Crossref] [ Google Scholar]
- Ghafouri-Fard S, Taheri M. The expression profile and role of non-coding RNAs in obesity. Eur J Pharmacol 2021; 892:173809. doi: 10.1016/j.ejphar.2020.173809 [Crossref] [ Google Scholar]
- Micha R, Kalantarian S, Wirojratana P, Byers T, Danaei G, Elmadfa I. Estimating the global and regional burden of suboptimal nutrition on chronic disease: methods and inputs to the analysis. Eur J Clin Nutr 2012; 66(1):119-29. doi: 10.1038/ejcn.2011.147 [Crossref] [ Google Scholar]
- Micha R, Shulkin ML, Peñalvo JL, Khatibzadeh S, Singh GM, Rao M. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One 2017; 12(4):e0175149. doi: 10.1371/journal.pone.0175149 [Crossref] [ Google Scholar]
- Willett WC, Stampfer MJ. Current evidence on healthy eating. Annu Rev Public Health 2013; 34:77-95. doi: 10.1146/annurev-publhealth-031811-124646 [Crossref] [ Google Scholar]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019; 393(10184):1958-72. doi: 10.1016/s0140-6736(19)30041-8 [Crossref] [ Google Scholar]
- Bleich S, Cutler D, Murray C, Adams A. Why is the developed world obese?. Annu Rev Public Health 2008; 29:273-95. doi: 10.1146/annurev.publhealth.29.020907.090954 [Crossref] [ Google Scholar]
- Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971-1975 to NHANES 1999-2002. Am J Clin Nutr 2006; 84(5):1215-23. doi: 10.1093/ajcn/84.5.1215 [Crossref] [ Google Scholar]
- Putnam J, Allshouse J, Kantor LS. US per capita food supply trends: more calories, refined carbohydrates, and fats. Food Review 2002; 25(3):2-15. doi: 10.22004/ag.econ.234624 [Crossref] [ Google Scholar]
- Office of the Surgeon General (US), Office of Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, National Institutes of Health. Publications and Reports of the Surgeon General. The Surgeon General’s Call to Action to Prevent and Decrease Overweight and Obesity. Rockville, MD: Office of the Surgeon General (US); 2001.
- Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004; 79(1):6-16. doi: 10.1093/ajcn/79.1.6 [Crossref] [ Google Scholar]
- Lin BH, Guthrie JF, Frazao E. Away-From-Home Foods Increasingly Important to Quality of American Diet. United States Department of Agriculture; 1999.
- Guthrie JF, Lin BH, Frazao E. Role of food prepared away from home in the American diet, 1977-78 versus 1994-96: changes and consequences. J Nutr Educ Behav 2002; 34(3):140-50. doi: 10.1016/s1499-4046(06)60083-3 [Crossref] [ Google Scholar]
- Stewart H, Blisard N, Bhuyan S, Nayga RM Jr. The Demand for Food Away from Home: Full-Service or Fast Food? United States Department of Agriculture; 2004.
- Hogan L. Food Demand in Australia: Trends and Issues 2018. Canberra: Commonwealth of Australia; 2018.
- Ledikwe JH, Ello-Martin JA, Rolls BJ. Portion sizes and the obesity epidemic. J Nutr 2005; 135(4):905-9. doi: 10.1093/jn/135.4.905 [Crossref] [ Google Scholar]
- Prentice AM, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev 2003; 4(4):187-94. doi: 10.1046/j.1467-789x.2003.00117.x [Crossref] [ Google Scholar]
- Glanz K, Basil M, Maibach E, Goldberg J, Snyder D. Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J Am Diet Assoc 1998; 98(10):1118-26. doi: 10.1016/s0002-8223(98)00260-0 [Crossref] [ Google Scholar]
- Bowman SA, Vinyard BT. Fast food consumption of US adults: impact on energy and nutrient intakes and overweight status. J Am Coll Nutr 2004; 23(2):163-8. doi: 10.1080/07315724.2004.10719357 [Crossref] [ Google Scholar]
- Paeratakul S, Ferdinand DP, Champagne CM, Ryan DH, Bray GA. Fast-food consumption among US adults and children: dietary and nutrient intake profile. J Am Diet Assoc 2003; 103(10):1332-8. doi: 10.1016/s0002-8223(03)01086-1 [Crossref] [ Google Scholar]
- Satia JA, Galanko JA, Siega-Riz AM. Eating at fast-food restaurants is associated with dietary intake, demographic, psychosocial and behavioural factors among African Americans in North Carolina. Public Health Nutr 2004; 7(8):1089-96. doi: 10.1079/phn2004662 [Crossref] [ Google Scholar]
- French SA, Harnack L, Jeffery RW. Fast food restaurant use among women in the Pound of Prevention study: dietary, behavioral and demographic correlates. Int J Obes Relat Metab Disord 2000; 24(10):1353-9. doi: 10.1038/sj.ijo.0801429 [Crossref] [ Google Scholar]
- Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity correlates of weight loss maintenance. Prev Chronic Dis 2008; 5(1):A11. [ Google Scholar]
- Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet 2014; 27 Suppl 2:255-62. doi: 10.1111/jhn.12141 [Crossref] [ Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 2001; 104(4):531-43. doi: 10.1016/s0092-8674(01)00240-9 [Crossref] [ Google Scholar]
-
Lakka TA, Bouchard C. Physical activity, obesity and cardiovascular diseases. Handb Exp Pharmacol. 2005(170):137-63. doi: 10.1007/3-540-27661-0_4.
- Montgomerie AM, Chittleborough CR, Taylor AW. Physical inactivity and incidence of obesity among South Australian adults. PLoS One 2014; 9(11):e112693. doi: 10.1371/journal.pone.0112693 [Crossref] [ Google Scholar]
- Madjd A, Taylor MA, Shafiei Neek L, Delavari A, Malekzadeh R, Macdonald IA. Effect of weekly physical activity frequency on weight loss in healthy overweight and obese women attending a weight loss program: a randomized controlled trial. Am J Clin Nutr 2016; 104(5):1202-8. doi: 10.3945/ajcn.116.136408 [Crossref] [ Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985; 100(2):126-31. [ Google Scholar]
- World Health Organization (WHO). WHO Guidelines on Physical Activity and Sedentary Behaviour: Web Annex Evidence Profiles. WHO; 2020.
- World Health Organization (WHO). Global Action Plan on Physical Activity 2018-2030: More Active People for a Healthier World. WHO; 2019.
- Bell JA, Hamer M, Batty GD, Singh-Manoux A, Sabia S, Kivimaki M. Combined effect of physical activity and leisure time sitting on long-term risk of incident obesity and metabolic risk factor clustering. Diabetologia 2014; 57(10):2048-56. doi: 10.1007/s00125-014-3323-8 [Crossref] [ Google Scholar]
- Pavey TG, Peeters GM, Gomersall SR, Brown WJ. Long-term effects of physical activity level on changes in healthy body mass index over 12 years in young adult women. Mayo Clin Proc 2016; 91(6):735-44. doi: 10.1016/j.mayocp.2016.03.008 [Crossref] [ Google Scholar]
- Chomistek AK, Henschel B, Eliassen AH, Mukamal KJ, Rimm EB. Frequency, type, and volume of leisure-time physical activity and risk of coronary heart disease in young women. Circulation 2016; 134(4):290-9. doi: 10.1161/circulationaha.116.021516 [Crossref] [ Google Scholar]
- Koolhaas CM, Dhana K, Golubic R, Schoufour JD, Hofman A, van Rooij FJ. Physical activity types and coronary heart disease risk in middle-aged and elderly persons: the Rotterdam study. Am J Epidemiol 2016; 183(8):729-38. doi: 10.1093/aje/kwv244 [Crossref] [ Google Scholar]
- Petersen CB, Grønbæk M, Helge JW, Thygesen LC, Schnohr P, Tolstrup JS. Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur J Epidemiol 2012; 27(2):91-9. doi: 10.1007/s10654-012-9656-z [Crossref] [ Google Scholar]
- Soares-Miranda L, Siscovick DS, Psaty BM, Longstreth WT Jr, Mozaffarian D. Physical activity and risk of coronary heart disease and stroke in older adults: the cardiovascular health study. Circulation 2016; 133(2):147-55. doi: 10.1161/circulationaha.115.018323 [Crossref] [ Google Scholar]
- Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank study. Circulation 2018; 137(24):2583-91. doi: 10.1161/circulationaha.117.032432 [Crossref] [ Google Scholar]
- Avery MD, Walker AJ. Acute effect of exercise on blood glucose and insulin levels in women with gestational diabetes. J Matern Fetal Med 2001; 10(1):52-8. doi: 10.1080/714904296 [Crossref] [ Google Scholar]
- Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010; 304(20):2253-62. doi: 10.1001/jama.2010.1710 [Crossref] [ Google Scholar]
- Nelson L, Jennings GL, Esler MD, Korner PI. Effect of changing levels of physical activity on blood-pressure and haemodynamics in essential hypertension. Lancet 1986; 2(8505):473-6. doi: 10.1016/s0140-6736(86)90354-5 [Crossref] [ Google Scholar]
- Silva DA, Naghavi M, Duncan BB, Schmidt MI, de Fatima Marinho de Souza M, Malta DC. Physical inactivity as risk factor for mortality by diabetes mellitus in Brazil in 1990, 2006, and 2016. Diabetol Metab Syndr 2019; 11:23. doi: 10.1186/s13098-019-0419-9 [Crossref] [ Google Scholar]
- AlQuaiz AM, Siddiqui AR, Kazi A, Batais MA, Al-Hazmi AM. Sedentary lifestyle and Framingham risk scores: a population-based study in Riyadh city, Saudi Arabia. BMC Cardiovasc Disord 2019; 19(1):88. doi: 10.1186/s12872-019-1048-9 [Crossref] [ Google Scholar]
- Gómez SF, Homs C, Wärnberg J, Medrano M, Gonzalez-Gross M, Gusi N. Study protocol of a population-based cohort investigating physical activity, sedentarism, lifestyles and obesity in Spanish youth: the PASOS study. BMJ Open 2020; 10(9):e036210. doi: 10.1136/bmjopen-2019-036210 [Crossref] [ Google Scholar]
- Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med 2020; 41(6):365-73. doi: 10.4082/kjfm.20.0165 [Crossref] [ Google Scholar]
- Kallio P, Pahkala K, Heinonen OJ, Tammelin TH, Pälve K, Hirvensalo M. Physical inactivity from youth to adulthood and adult cardiometabolic risk profile. Prev Med 2021; 145:106433. doi: 10.1016/j.ypmed.2021.106433 [Crossref] [ Google Scholar]
- Ding L, Liang Y, Tan ECK, Hu Y, Zhang C, Liu Y. Smoking, heavy drinking, physical inactivity, and obesity among middle-aged and older adults in China: cross-sectional findings from the baseline survey of CHARLS 2011-2012. BMC Public Health 2020; 20(1):1062. doi: 10.1186/s12889-020-08625-5 [Crossref] [ Google Scholar]
- Juna CF, Cho YH, Joung H. Low elevation and physical inactivity are associated with a higher prevalence of metabolic syndrome in ecuadorian adults: a national cross-sectional study. Diabetes Metab Syndr Obes 2020; 13:2217-26. doi: 10.2147/dmso.S253099 [Crossref] [ Google Scholar]
- Mainous AG 3rd, Tanner RJ, Rahmanian KP, Jo A, Carek PJ. Effect of sedentary lifestyle on cardiovascular disease risk among healthy adults with body mass indexes 185 to 299 kg/m2. Am J Cardiol 2019; 123(5):764-8. doi: 10.1016/j.amjcard.2018.11.043 [Crossref] [ Google Scholar]
- Lippi G, Henry BM, Sanchis-Gomar F. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). Eur J Prev Cardiol 2020; 27(9):906-8. doi: 10.1177/2047487320916823 [Crossref] [ Google Scholar]
- Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc 2009; 41(5):998-1005. doi: 10.1249/MSS.0b013e3181930355 [Crossref] [ Google Scholar]
- Kohl HW 3rd, Craig CL, Lambert EV, Inoue S, Alkandari JR, Leetongin G. The pandemic of physical inactivity: global action for public health. Lancet 2012; 380(9838):294-305. doi: 10.1016/s0140-6736(12)60898-8 [Crossref] [ Google Scholar]
- Ozemek C, Lavie CJ, Rognmo Ø. Global physical activity levels - need for intervention. Prog Cardiovasc Dis 2019; 62(2):102-7. doi: 10.1016/j.pcad.2019.02.004 [Crossref] [ Google Scholar]
-
Jochem C, Schmid D, Leitzmann MF. Introduction to sedentary behaviour epidemiology. In: Leitzmann MF, Jochem C, Schmid D, eds. Sedentary Behaviour Epidemiology. Cham: Springer; 2018. p. 3-29. doi: 10.1007/978-3-319-61552-3_1.
- Panahi S, Tremblay A. Sedentariness and health: is sedentary behavior more than just physical inactivity?. Front Public Health 2018; 6:258. doi: 10.3389/fpubh.2018.00258 [Crossref] [ Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell 2001; 104(4):531-43. doi: 10.1016/s0092-8674(01)00240-9 [Crossref] [ Google Scholar]
- Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M. Energy balance and obesity: what are the main drivers?. ancer Causes Control 2017; 28(3):247-58. doi: 10.1007/s10552-017-0869-z [Crossref] [ Google Scholar]
- Colley RC, Hills AP, King NA, Byrne NM. Exercise-induced energy expenditure: implications for exercise prescription and obesity. Patient Educ Couns 2010; 79(3):327-32. doi: 10.1016/j.pec.2010.03.001 [Crossref] [ Google Scholar]
- Martin AR, Murphy C, Hofmann H, Drenowatz C, Wallmann-Sperlich B, Sperlich B, et al. The impact of prescribed exercise on non-exercise activity during short-term caloric restriction. Nutrients. 2020.
- Levine JA. Non-exercise activity thermogenesis (NEAT). Best Pract Res Clin Endocrinol Metab 2002; 16(4):679-702. doi: 10.1053/beem.2002.0227 [Crossref] [ Google Scholar]
- Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol 2013; 4:90. doi: 10.3389/fphys.2013.00090 [Crossref] [ Google Scholar]
- Wells GD, Selvadurai H, Tein I. Bioenergetic provision of energy for muscular activity. Paediatr Respir Rev 2009; 10(3):83-90. doi: 10.1016/j.prrv.2009.04.005 [Crossref] [ Google Scholar]
- Hargreaves M. Skeletal muscle metabolism during exercise in humans. Clin Exp Pharmacol Physiol 2000; 27(3):225-8. doi: 10.1046/j.1440-1681.2000.03225.x [Crossref] [ Google Scholar]
- Muscella A, Stefàno E, Lunetti P, Capobianco L, Marsigliante S. The regulation of fat metabolism during aerobic exercise. Biomolecules 2020; 10(12):1699. doi: 10.3390/biom10121699 [Crossref] [ Google Scholar]
- Melzer K. Carbohydrate and fat utilization during rest and physical activity. E Spen Eur E J Clin Nutr Metab 2011; 6(2):e45-52. doi: 10.1016/j.eclnm.2011.01.005 [Crossref] [ Google Scholar]
- Maffeis C, Castellani M. Physical activity: an effective way to control weight in children?. Nutr Metab Cardiovasc Dis 2007; 17(5):394-408. doi: 10.1016/j.numecd.2006.08.006 [Crossref] [ Google Scholar]
- Forbes GB. Lean body mass-body fat interrelationships in humans. Nutr Rev 1987; 45(8):225-31. doi: 10.1111/j.1753-4887.1987.tb02684.x [Crossref] [ Google Scholar]
- Ara I, Vicente-Rodriguez G, Perez-Gomez J, Jimenez-Ramirez J, Serrano-Sanchez JA, Dorado C. Influence of extracurricular sport activities on body composition and physical fitness in boys: a 3-year longitudinal study. Int J Obes (Lond) 2006; 30(7):1062-71. doi: 10.1038/sj.ijo.0803303 [Crossref] [ Google Scholar]
- Kreider RB. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med 1999; 27(2):97-110. doi: 10.2165/00007256-199927020-00003 [Crossref] [ Google Scholar]
- Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc 2003; 62(3):621-34. doi: 10.1079/pns2003282 [Crossref] [ Google Scholar]
- Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 2010; 92(6):1369-77. doi: 10.3945/ajcn.2010.29885 [Crossref] [ Google Scholar]
- Wasse LK, Sunderland C, King JA, Batterham RL, Stensel DJ. Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J Appl Physiol (1985) 2012; 112(4):552-9. doi: 10.1152/japplphysiol.00090.2011 [Crossref] [ Google Scholar]
- Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 2010; 57 Suppl 2:36-42. doi: 10.1159/000322702 [Crossref] [ Google Scholar]
- Prado WL, Lofrano-Prado MC, Oyama LM, Cardel M, Gomes PP, Andrade ML. Effect of a 12-week low vs high intensity aerobic exercise training on appetite-regulating hormones in obese adolescents: a randomized exercise intervention study. Pediatr Exerc Sci 2015; 27(4):510-7. doi: 10.1123/pes.2015-0018 [Crossref] [ Google Scholar]
- Annesi JJ, Whitaker AC. Psychological factors associated with weight loss in obese and severely obese women in a behavioral physical activity intervention. Health Educ Behav 2010; 37(4):593-606. doi: 10.1177/1090198109331671 [Crossref] [ Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Health Nutr 1999; 2(3a):411-8. doi: 10.1017/s1368980099000567 [Crossref] [ Google Scholar]
- Maugeri G, Castrogiovanni P, Battaglia G, Pippi R, D’Agata V, Palma A. The impact of physical activity on psychological health during COVID-19 pandemic in Italy. Heliyon 2020; 6(6):e04315. doi: 10.1016/j.heliyon.2020.e04315 [Crossref] [ Google Scholar]
- Smith KE, O’Connor SM, Mason TB, Wang S, Dzubur E, Crosby RD. Associations between objective physical activity and emotional eating among adiposity-discordant siblings using ecological momentary assessment and accelerometers. Pediatr Obes 2021; 16(3):e12720. doi: 10.1111/ijpo.12720 [Crossref] [ Google Scholar]
- King JE, Jebeile H, Garnett SP, Baur LA, Paxton SJ, Gow ML. Physical activity based pediatric obesity treatment, depression, self-esteem and body image: a systematic review with meta-analysis. Ment Health Phys Act 2020; 19:100342. doi: 10.1016/j.mhpa.2020.100342 [Crossref] [ Google Scholar]
- Catenacci VA, Wyatt HR. The role of physical activity in producing and maintaining weight loss. Nat Clin Pract Endocrinol Metab 2007; 3(7):518-29. doi: 10.1038/ncpendmet0554 [Crossref] [ Google Scholar]
-
Özdamar Ünal G. Psychiatric aspects of obesity, behavior and cognitive behavior therapy in treatment. J Cogn Behav Psychother Res. 2018; 7(1):31-41. doi: 10.5455/jcbpr.268112.
- Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol (Lausanne) 2021; 12:706978. doi: 10.3389/fendo.2021.706978 [Crossref] [ Google Scholar]
- Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology 2007; 132(6):2226-38. doi: 10.1053/j.gastro.2007.03.051 [Crossref] [ Google Scholar]
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am 2011; 34(4):841-59. doi: 10.1016/j.psc.2011.08.006 [Crossref] [ Google Scholar]
- Byrne SM. Psychological aspects of weight maintenance and relapse in obesity. J Psychosom Res 2002; 53(5):1029-36. doi: 10.1016/s0022-3999(02)00487-7 [Crossref] [ Google Scholar]
- Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev 2013; 14(11):906-18. doi: 10.1111/obr.12052 [Crossref] [ Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67(3):220-9. doi: 10.1001/archgenpsychiatry.2010.2 [Crossref] [ Google Scholar]
- Kalarchian MA, Marcus MD. Psychiatric comorbidity of childhood obesity. Int Rev Psychiatry 2012; 24(3):241-6. doi: 10.3109/09540261.2012.678818 [Crossref] [ Google Scholar]
- Foroughan M, Namjoo S, Mirzaei M, Ghaedamini Harouni G, Abbasian M, Afaridoun K. Anthropometrics and mental health among people with and without type 2 diabetes. Int J Aging 2024; 2(1):e6. doi: 10.34172/ija.2024.e6 [Crossref] [ Google Scholar]
- Moreira RO, Marca KF, Appolinario JC, Coutinho WF. Increased waist circumference is associated with an increased prevalence of mood disorders and depressive symptoms in obese women. Eat Weight Disord 2007; 12(1):35-40. doi: 10.1007/bf03327770 [Crossref] [ Google Scholar]
- Labad J, Price JF, Strachan MW, Fowkes FG, Ding J, Deary IJ. Symptoms of depression but not anxiety are associated with central obesity and cardiovascular disease in people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetologia 2010; 53(3):467-71. doi: 10.1007/s00125-009-1628-9 [Crossref] [ Google Scholar]
- Whitaker BN, Fisher PL, Jambhekar S, Com G, Razzaq S, Thompson JE. Impact of degree of obesity on sleep, quality of life, and depression in youth. J Pediatr Health Care 2018; 32(2):e37-44. doi: 10.1016/j.pedhc.2017.09.008 [Crossref] [ Google Scholar]
- Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005-2010. NCHS Data Brief. 2014(167):1-8.
- American Psychiatric Association (APA). Depressive Disorders: DSM-5® Selections. APA; 2015.
- Caroleo M, Carbone EA, Primerano A, Foti D, Brunetti A, Segura-Garcia C. The role of hormonal, metabolic and inflammatory biomarkers on sleep and appetite in drug free patients with major depression: a systematic review. J Affect Disord 2019; 250:249-59. doi: 10.1016/j.jad.2019.03.015 [Crossref] [ Google Scholar]
- Moitra P, Madan J, Shaikh NI. Eating habits and sleep patterns of adolescents with depression symptoms in Mumbai, India. Matern Child Nutr 2020; 16(Suppl 3):e12998. doi: 10.1111/mcn.12998 [Crossref] [ Google Scholar]
- Mills JG, Larkin TA, Deng C, Thomas SJ. Cortisol in relation to problematic eating behaviours, adiposity and symptom profiles in major depressive disorder. Compr Psychoneuroendocrinol 2021; 7:100067. doi: 10.1016/j.cpnec.2021.100067 [Crossref] [ Google Scholar]
- Witek K, Wydra K, Filip M. A high-sugar diet consumption, metabolism and health impacts with a focus on the development of substance use disorder: a narrative review. Nutrients 2022; 14(14):2940. doi: 10.3390/nu14142940 [Crossref] [ Google Scholar]
- Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci 2013; 7:177. doi: 10.3389/fnins.2013.00177 [Crossref] [ Google Scholar]
- Mills JG, Larkin TA, Deng C, Thomas SJ. Weight gain in major depressive disorder: linking appetite and disordered eating to leptin and ghrelin. Psychiatry Res 2019; 279:244-51. doi: 10.1016/j.psychres.2019.03.017 [Crossref] [ Google Scholar]
- Simmons WK, Burrows K, Avery JA, Kerr KL, Taylor A, Bodurka J. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol Psychiatry 2020; 25(7):1457-68. doi: 10.1038/s41380-018-0093-6 [Crossref] [ Google Scholar]
- Akter S, Pham NM, Nanri A, Kurotani K, Kuwahara K, Jacka FN. Association of serum leptin and ghrelin with depressive symptoms in a Japanese working population: a cross-sectional study. BMC Psychiatry 2014; 14:203. doi: 10.1186/1471-244x-14-203 [Crossref] [ Google Scholar]
- Chopra AM, Bachu R, Peterson MJ. Depressive disorders. In: Chopra A, Das P, Doghramji K, eds. Management of Sleep Disorders in Psychiatry. Oxford University Press; 2020.
- Papatriantafyllou E, Efthymiou D, Zoumbaneas E, Popescu CA, Vassilopoulou E. Sleep deprivation: effects on weight loss and weight loss maintenance. Nutrients 2022; 14(8):1549. doi: 10.3390/nu14081549 [Crossref] [ Google Scholar]
- Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun 2013; 4:2259. doi: 10.1038/ncomms3259 [Crossref] [ Google Scholar]
- Axelsson J, Ingre M, Kecklund G, Lekander M, Wright KP, Sundelin T. Sleepiness as motivation: a potential mechanism for how sleep deprivation affects behavior. Sleep 2020; 43(6):zsz291. doi: 10.1093/sleep/zsz291 [Crossref] [ Google Scholar]
- Qiu W, Cai X, Zheng C, Qiu S, Ke H, Huang Y. Update on the relationship between depression and neuroendocrine metabolism. Front Neurosci 2021; 15:728810. doi: 10.3389/fnins.2021.728810 [Crossref] [ Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition 2007; 23(11-12):887-94. doi: 10.1016/j.nut.2007.08.008 [Crossref] [ Google Scholar]
- Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am 2016; 45(3):677-88. doi: 10.1016/j.ecl.2016.04.016 [Crossref] [ Google Scholar]
- Lindberg L, Hagman E, Danielsson P, Marcus C, Persson M. Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med 2020; 18(1):30. doi: 10.1186/s12916-020-1498-z [Crossref] [ Google Scholar]
- Singh M. Mood, food, and obesity. Front Psychol 2014; 5:925. doi: 10.3389/fpsyg.2014.00925 [Crossref] [ Google Scholar]
- Alhussain MH, Macdonald IA, Taylor MA. Irregular meal-pattern effects on energy expenditure, metabolism, and appetite regulation: a randomized controlled trial in healthy normal-weight women. Am J Clin Nutr 2016; 104(1):21-32. doi: 10.3945/ajcn.115.125401 [Crossref] [ Google Scholar]
- Yau YH, Potenza MN. Stress and eating behaviors. Minerva Endocrinol 2013; 38(3):255-67. [ Google Scholar]
- Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Med 2014; 44(1):81-121. doi: 10.1007/s40279-013-0090-5 [Crossref] [ Google Scholar]
- Fava M. Weight gain and antidepressants. J Clin Psychiatry 2000; 61 Suppl 11:37-41. [ Google Scholar]
- Alonso-Pedrero L, Bes-Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: a systematic review. Obes Rev 2019; 20(12):1680-90. doi: 10.1111/obr.12934 [Crossref] [ Google Scholar]
- Berken GH, Weinstein DO, Stern WC. Weight gain A side-effect of tricyclic antidepressants. J Affect Disord 1984; 7(2):133-8. doi: 10.1016/0165-0327(84)90031-4 [Crossref] [ Google Scholar]
- Pyle E. A Review on the Side Effect of Weight Gain in Response to Selective Serotonin Reuptake Inhibitors, Monoamine Oxidase Inhibitors, and Benzodiazepines. 2020. Available from: https://webs.wofford.edu/pittmandw/psy451/fall20ep.pdf.
- Wang K, Wu C, Yao Y, Zhang S, Xie Y, Shi K. Association between socio-economic factors and the risk of overweight and obesity among Chinese adults: a retrospective cross-sectional study from the China Health and Nutrition Survey. Glob Health Res Policy 2022; 7(1):41. doi: 10.1186/s41256-022-00274-y [Crossref] [ Google Scholar]
- Anekwe CV, Jarrell AR, Townsend MJ, Gaudier GI, Hiserodt JM, Stanford FC. Socioeconomics of obesity. Curr Obes Rep 2020; 9(3):272-9. doi: 10.1007/s13679-020-00398-7 [Crossref] [ Google Scholar]
- Corner WP. Socioeconomic Factors Impacting Obesity Care: Identifying and Addressing Challenges in Clinical Practice. Bariatric Times; 2018.
- Wang Y, Beydoun MA. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007; 29:6-28. doi: 10.1093/epirev/mxm007 [Crossref] [ Google Scholar]
- Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev 2012; 13(11):1067-79. doi: 10.1111/j.1467-789X.2012.01017.x [Crossref] [ Google Scholar]
- Fan JX, Wen M, Li K. Associations between obesity and neighborhood socioeconomic status: variations by gender and family income status. SSM Popul Health 2020; 10:100529. doi: 10.1016/j.ssmph.2019.100529 [Crossref] [ Google Scholar]
- Luiggi M, Rey O, Travert M, Griffet J. Overweight and obesity by school socioeconomic composition and adolescent socioeconomic status: a school-based study. BMC Public Health 2021; 21(1):1837. doi: 10.1186/s12889-021-11752-2 [Crossref] [ Google Scholar]
- Monsivais P, Aggarwal A, Drewnowski A. Are socio-economic disparities in diet quality explained by diet cost?. J Epidemiol Community Health 2012; 66(6):530-5. doi: 10.1136/jech.2010.122333 [Crossref] [ Google Scholar]
- Crawford PB, Webb KL. Unraveling the paradox of concurrent food insecurity and obesity. Am J Prev Med 2011; 40(2):274-5. doi: 10.1016/j.amepre.2010.11.003 [Crossref] [ Google Scholar]
- Rahimi Mamaghani A, Abdi F, Abbasalizad Farhangi M. Food insecurity prevalence and its demographic, anthropometric, and nutritional determinants among overweight and obese patients with diabetes and coronary artery disease. Int J Drug Res Clin 2024; 2:e15. doi: 10.34172/ijdrc.2024.e15 [Crossref] [ Google Scholar]
- Abdi F, Rahimi Mamaghani A, Abbasalizad Farhangi M. Food insecurity among overweight/obese patients with non-alcoholic fatty liver disease (NAFLD) in Iran: demographic and anthropometric factors (a cross-sectional study). Int J Drug Res Clin 2024; 2:e14. doi: 10.34172/ijdrc.2024.e14 [Crossref] [ Google Scholar]
- Devereux-Fitzgerald A, Powell R, French DP. The acceptability of physical activity to older adults living in lower socioeconomic status areas: a multi-perspective study. Int J Environ Res Public Health 2021; 18(22):11784. doi: 10.3390/ijerph182211784 [Crossref] [ Google Scholar]
- Larson NI, Story MT. Food insecurity and weight status among US children and families: a review of the literature. Am J Prev Med 2011; 40(2):166-73. doi: 10.1016/j.amepre.2010.10.028 [Crossref] [ Google Scholar]
- Pruchno R, Wilson-Genderson M, Gupta AK. Neighborhood food environment and obesity in community-dwelling older adults: individual and neighborhood effects. Am J Public Health 2014; 104(5):924-9. doi: 10.2105/ajph.2013.301788 [Crossref] [ Google Scholar]
- Jin H, Lu Y. Evaluating consumer nutrition environment in food deserts and food swamps. Int J Environ Res Public Health 2021; 18(5):2675. doi: 10.3390/ijerph18052675 [Crossref] [ Google Scholar]
- Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci U S A 2014; 111(4):1338-42. doi: 10.1073/pnas.1321355110 [Crossref] [ Google Scholar]
- Tajdar D, Schäfer I, Lühmann D, Fertmann R, Steinberg T, van den Bussche H. The link between health literacy and three conditions of metabolic syndrome: obesity, diabetes and hypertension. Diabetes Metab Syndr Obes 2022; 15:1639-50. doi: 10.2147/dmso.S363823 [Crossref] [ Google Scholar]
- Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995-2015. Arch Public Health 2020; 78:20. doi: 10.1186/s13690-020-00402-5 [Crossref] [ Google Scholar]
- Aceves-Martins M, López-Cruz L, García-Botello M, Godina-Flores NL, Gutierrez-Gómez YY, Moreno-García CF. Cultural factors related to childhood and adolescent obesity in Mexico: a systematic review of qualitative studies. Obes Rev 2022; 23(9):e13461. doi: 10.1111/obr.13461 [Crossref] [ Google Scholar]
- Poobalan AS, Aucott LS, Clarke A, Smith WC. Physical activity attitudes, intentions and behaviour among 18-25 year olds: a mixed method study. BMC Public Health 2012; 12:640. doi: 10.1186/1471-2458-12-640 [Crossref] [ Google Scholar]
- Dao MC, Thiron S, Messer E, Sergeant C, Sévigné A, Huart C. Cultural influences on the regulation of energy intake and obesity: a qualitative study comparing food customs and attitudes to eating in adults from France and the United States. Nutrients 2020; 13(1):63. doi: 10.3390/nu13010063 [Crossref] [ Google Scholar]
- Voorhees CC, Murray D, Welk G, Birnbaum A, Ribisl KM, Johnson CC. The role of peer social network factors and physical activity in adolescent girls. Am J Health Behav 2005; 29(2):183-90. doi: 10.5993/ajhb.29.2.9 [Crossref] [ Google Scholar]
- Powell K, Wilcox J, Clonan A, Bissell P, Preston L, Peacock M. The role of social networks in the development of overweight and obesity among adults: a scoping review. BMC Public Health 2015; 15:996. doi: 10.1186/s12889-015-2314-0 [Crossref] [ Google Scholar]
- Serrano Fuentes N, Rogers A, Portillo MC. Social network influences and the adoption of obesity-related behaviours in adults: a critical interpretative synthesis review. BMC Public Health 2019; 19(1):1178. doi: 10.1186/s12889-019-7467-9 [Crossref] [ Google Scholar]
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 1999; 282(16):1547-53. doi: 10.1001/jama.282.16.1547 [Crossref] [ Google Scholar]
- Lycett K, Juonala M, Magnussen CG, Norrish D, Mensah FK, Liu R. Body mass index from early to late childhood and cardiometabolic measurements at 11 to 12 years. Pediatrics 2020; 146(2):e20193666. doi: 10.1542/peds.2019-3666 [Crossref] [ Google Scholar]
- Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis 2014; 56(4):391-400. doi: 10.1016/j.pcad.2013.09.003 [Crossref] [ Google Scholar]
- Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med 2007; 4(8):436-43. doi: 10.1038/ncpcardio0943 [Crossref] [ Google Scholar]
- Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med 1976; 60(5):645-53. doi: 10.1016/0002-9343(76)90499-x [Crossref] [ Google Scholar]
- Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging 2013; 6(1):142-52. doi: 10.1161/circimaging.111.964627 [Crossref] [ Google Scholar]
- Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis 2016; 58(4):393-400. doi: 10.1016/j.pcad.2015.12.003 [Crossref] [ Google Scholar]
- Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog Cardiovasc Dis 2016; 58(5):537-47. doi: 10.1016/j.pcad.2016.01.008 [Crossref] [ Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270(45):26746-9. doi: 10.1074/jbc.270.45.26746 [Crossref] [ Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372(6505):425-32. doi: 10.1038/372425a0 [Crossref] [ Google Scholar]
- Ren J. Leptin and hyperleptinemia - from friend to foe for cardiovascular function. J Endocrinol 2004; 181(1):1-10. doi: 10.1677/joe.0.1810001 [Crossref] [ Google Scholar]
- Martínez-Martínez E, Jurado-López R, Cervantes-Escalera P, Cachofeiro V, Miana M. Leptin, a mediator of cardiac damage associated with obesity. Horm Mol Biol Clin Investig 2014; 18(1):3-14. doi: 10.1515/hmbci-2013-0060 [Crossref] [ Google Scholar]
- Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension 2005; 45(6):1031-4. doi: 10.1161/01.Hyp.0000165683.09053.02 [Crossref] [ Google Scholar]
- Lee CH, Lui DT, Cheung CY, Fong CH, Yuen MM, Chow WS. Higher circulating adiponectin concentrations predict incident cancer in type 2 diabetes - the adiponectin paradox. J Clin Endocrinol Metab 2020; 105(4):dgaa075. doi: 10.1210/clinem/dgaa075 [Crossref] [ Google Scholar]
- Baker JF, Newman AB, Kanaya A, Leonard MB, Zemel B, Miljkovic I. The adiponectin paradox in the elderly: associations with body composition, physical functioning, and mortality. J Gerontol A Biol Sci Med Sci 2019; 74(2):247-53. doi: 10.1093/gerona/gly017 [Crossref] [ Google Scholar]
- Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg?. Diabetes Care 2008; 31 Suppl 2(7):S303-9. doi: 10.2337/dc08-s272 [Crossref] [ Google Scholar]
- Akinnusi ME, Saliba R, Porhomayon J, El-Solh AA. Sleep disorders in morbid obesity. Eur J Intern Med 2012; 23(3):219-26. doi: 10.1016/j.ejim.2011.10.016 [Crossref] [ Google Scholar]
- Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc 2008; 5(2):185-92. doi: 10.1513/pats.200708-137MG [Crossref] [ Google Scholar]
- Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA 2013; 310(3):280-8. doi: 10.1001/jama.2013.7833 [Crossref] [ Google Scholar]
- Reis JP, Allen N, Gunderson EP, Lee JM, Lewis CE, Loria CM. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity (Silver Spring) 2015; 23(4):879-85. doi: 10.1002/oby.21023 [Crossref] [ Google Scholar]
- Tanamas SK, Wong E, Backholer K, Abdullah A, Wolfe R, Barendregt J. Duration of obesity and incident hypertension in adults from the Framingham Heart Study. J Hypertens 2015; 33(3):542-5. doi: 10.1097/hjh.0000000000000441 [Crossref] [ Google Scholar]
- Dowd JB, Zajacova A. Long-term obesity and cardiovascular, inflammatory, and metabolic risk in US adults. Am J Prev Med 2014; 46(6):578-84. doi: 10.1016/j.amepre.2014.01.016 [Crossref] [ Google Scholar]
- Lega IC, Lipscombe LL. Diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev 2020; 41(1):bnz014. doi: 10.1210/endrev/bnz014 [Crossref] [ Google Scholar]
- Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2018; 6(2):95-104. doi: 10.1016/s2213-8587(17)30366-2 [Crossref] [ Google Scholar]
- Chadid S, Singer MR, Kreger BE, Bradlee ML, Moore LL. Midlife weight gain is a risk factor for obesity-related cancer. Br J Cancer 2018; 118(12):1665-71. doi: 10.1038/s41416-018-0106-x [Crossref] [ Google Scholar]
- Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology 2009; 150(6):2537-42. doi: 10.1210/en.2009-0070 [Crossref] [ Google Scholar]
- Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat 2013; 137(1):307-14. doi: 10.1007/s10549-012-2339-3 [Crossref] [ Google Scholar]
- Schoemaker MJ, Nichols HB, Wright LB, Brook MN, Jones ME, O’Brien KM. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol 2018; 4(11):e181771. doi: 10.1001/jamaoncol.2018.1771 [Crossref] [ Google Scholar]
- Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010; 123(3):627-35. doi: 10.1007/s10549-010-0990-0 [Crossref] [ Google Scholar]
- Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014; 25(10):1901-14. doi: 10.1093/annonc/mdu042 [Crossref] [ Google Scholar]
- Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019; 16(12):713-32. doi: 10.1038/s41575-019-0189-8 [Crossref] [ Google Scholar]
- Nam GE, Baek SJ, Choi HB, Han K, Kwak JM, Kim J. Association between abdominal obesity and incident colorectal cancer: a nationwide cohort study in Korea. Cancers (Basel) 2020; 12(6):1368. doi: 10.3390/cancers12061368 [Crossref] [ Google Scholar]
- Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 2019; 5(1):37-44. doi: 10.1001/jamaoncol.2018.4280 [Crossref] [ Google Scholar]
- Charneco E, Ortiz AP, Venegas-Ríos HL, Romaguera J, Umpierre S. Clinic-based case-control study of the association between body mass index and endometrial cancer in Puerto Rican women. P R Health Sci J 2010; 29(3):272-8. [ Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007; 335(7630):1134. doi: 10.1136/bmj.39367.495995.AE [Crossref] [ Google Scholar]
- Chaves GV, de Almeida Simao T, Pinto LF, Moreira MA, Bergmann A, Chaves CB. Overweight and obesity do not determine worst prognosis in endometrioid endometrial carcinoma. Arch Gynecol Obstet 2019; 300(6):1671-7. doi: 10.1007/s00404-019-05281-y [Crossref] [ Google Scholar]
- Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin 2019; 69(2):88-112. doi: 10.3322/caac.21499 [Crossref] [ Google Scholar]
- Bray GA. Handbook of Obesity--Volume 1: Epidemiology, Etiology, and Physiopathology. CRC Press; 2014.
- West KM, Kalbfleisch JM. Glucose tolerance, nutrition, and diabetes in Uruguay, Venezuela, Malaya, and East Pakistan. Diabetes 1966; 15(1):9-18. doi: 10.2337/diab.15.1.9 [Crossref] [ Google Scholar]
- Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes 2000; 49(6):883-8. doi: 10.2337/diabetes.49.6.883 [Crossref] [ Google Scholar]
- Must A, McKeown NM. The disease burden associated with overweight and obesity. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al, eds. In: Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000.
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289(1):76-9. doi: 10.1001/jama.289.1.76 [Crossref] [ Google Scholar]
- Ohlson LO, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L. The influence of body fat distribution on the incidence of diabetes mellitus 135 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985; 34(10):1055-8. doi: 10.2337/diab.34.10.1055 [Crossref] [ Google Scholar]
- Lundgren H, Bengtsson C, Blohme G, Lapidus L, Sjöström L. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes 1989; 13(4):413-23. [ Google Scholar]
- Haffner SM, Stern MP, Hazuda HP, Pugh J, Patterson JK. Do upper-body and centralized adiposity measure different aspects of regional body-fat distribution? Relationship to non-insulin-dependent diabetes mellitus, lipids, and lipoproteins. Diabetes 1987; 36(1):43-51. doi: 10.2337/diab.36.1.43 [Crossref] [ Google Scholar]
- Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000; 23(4):465-71. doi: 10.2337/diacare.23.4.465 [Crossref] [ Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444(7121):840-6. doi: 10.1038/nature05482 [Crossref] [ Google Scholar]
- Abuyassin B, Laher I. Obesity-linked diabetes in the Arab world: a review. East Mediterr Health J 2015; 21(6):420-39. doi: 10.26719/2015.21.420 [Crossref] [ Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008; 29(24):2959-71. doi: 10.1093/eurheartj/ehn387 [Crossref] [ Google Scholar]
- Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond) 2005; 109(3):243-56. doi: 10.1042/cs20050078 [Crossref] [ Google Scholar]
- Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F. The Metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects prospective data from the Verona Diabetes Complications Study. Diabet Med 2004; 21(1):52-8. doi: 10.1046/j.1464-5491.2003.01068.x [Crossref] [ Google Scholar]
- Masuo K, Rakugi H, Ogihara T, Esler MD, Lambert GW. Cardiovascular and renal complications of type 2 diabetes in obesity: role of sympathetic nerve activity and insulin resistance. Curr Diabetes Rev 2010; 6(2):58-67. doi: 10.2174/157339910790909396 [Crossref] [ Google Scholar]
- Raz I. Complex impact of obesity on type 2 diabetes. Isr Med Assoc J 2005; 7(6):402-3. [ Google Scholar]
- Ehtisham S, Barrett TG, Shaw NJ. Type 2 diabetes mellitus in UK children--an emerging problem. Diabet Med 2000; 17(12):867-71. doi: 10.1046/j.1464-5491.2000.00409.x [Crossref] [ Google Scholar]
- Punnose J, Agarwal MM, Bin-Uthman S. Type 2 diabetes mellitus among children and adolescents in Al-Ain: a case series. East Mediterr Health J 2005; 11(4):788-97. [ Google Scholar]
- Osman HA, Elsadek N, Abdullah MA. Type 2 diabetes in Sudanese children and adolescents. Sudan J Paediatr 2013; 13(2):17-23. [ Google Scholar]
- Conway B, Miller RG, Costacou T, Fried L, Kelsey S, Evans RW. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med 2010; 27(4):398-404. doi: 10.1111/j.1464-5491.2010.02956.x [Crossref] [ Google Scholar]
- Baskaran C, Volkening LK, Diaz M, Laffel LM. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the diabetes control and complications trial. Pediatr Diabetes 2015; 16(4):263-70. doi: 10.1111/pedi.12166 [Crossref] [ Google Scholar]
- Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: the T1D exchange clinic registry. Diabetes Res Clin Pract 2017; 126:68-78. doi: 10.1016/j.diabres.2017.01.012 [Crossref] [ Google Scholar]
- Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2015; 22(4):277-82. doi: 10.1097/med.0000000000000170 [Crossref] [ Google Scholar]
- Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 2018; 39(5):629-63. doi: 10.1210/er.2017-00191 [Crossref] [ Google Scholar]
- Robinson HE, O’Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 2005; 106(6):1357-64. doi: 10.1097/01.Aog.0000188387.88032.41 [Crossref] [ Google Scholar]
- Sikaris KA. The clinical biochemistry of obesity. Clin Biochem Rev 2004; 25(3):165-81. [ Google Scholar]
- Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 2003; 41(4):733-41. doi: 10.1016/s0272-6386(03)00020-9 [Crossref] [ Google Scholar]
- Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 2008; 52(1):39-48. doi: 10.1053/j.ajkd.2008.03.003 [Crossref] [ Google Scholar]
- Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidney Dis 2005; 46(4):587-94. doi: 10.1053/j.ajkd.2005.06.007 [Crossref] [ Google Scholar]
- Chang A, Van Horn L, Jacobs DR, Jr Jr, Liu K, Muntner P, Newsome B. Lifestyle-related factors, obesity, and incident microalbuminuria: the CARDIA (coronary artery risk development in young adults) study. Am J Kidney Dis 2013; 62(2):267-75. doi: 10.1053/j.ajkd.2013.02.363 [Crossref] [ Google Scholar]
- Shamekh A, Tahmasbi F, Mousavi SE, Nejadghaderi A, Sullman MJ, Kolahi AA. Burden of chronic kidney disease for adults 70 years and older in Iran during 1990-2021. Int J Aging 2024; 2(1):e12. doi: 10.34172/ija.2024.e12 [Crossref] [ Google Scholar]
- Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol 2006; 17(6):1695-702. doi: 10.1681/asn.2005060638 [Crossref] [ Google Scholar]
- Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 2005; 46(5):871-80. doi: 10.1053/j.ajkd.2005.08.015 [Crossref] [ Google Scholar]
- Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol 2015; 3(9):704-14. doi: 10.1016/s2213-8587(15)00128-x [Crossref] [ Google Scholar]
- Munkhaugen J, Lydersen S, Widerøe TE, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis 2009; 54(4):638-46. doi: 10.1053/j.ajkd.2009.03.023 [Crossref] [ Google Scholar]
- Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 2004; 65(5):1870-6. doi: 10.1111/j.1523-1755.2004.00582.x [Crossref] [ Google Scholar]
- Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K. Body mass index in 12 million adolescents and risk for end-stage renal disease. Arch Intern Med 2012; 172(21):1644-50. doi: 10.1001/2013.jamainternmed.85 [Crossref] [ Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006; 144(1):21-8. doi: 10.7326/0003-4819-144-1-200601030-00006 [Crossref] [ Google Scholar]
- Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 2014; 25(9):2088-96. doi: 10.1681/asn.2013070754 [Crossref] [ Google Scholar]
- Wang M, Wang Z, Chen Y, Dong Y. Kidney damage caused by obesity and its feasible treatment drugs. Int J Mol Sci 2022; 23(2):747. doi: 10.3390/ijms23020747 [Crossref] [ Google Scholar]
- Tsuboi N, Koike K, Hirano K, Utsunomiya Y, Kawamura T, Hosoya T. Clinical features and long-term renal outcomes of Japanese patients with obesity-related glomerulopathy. Clin Exp Nephrol 2013; 17(3):379-85. doi: 10.1007/s10157-012-0719-y [Crossref] [ Google Scholar]
- D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 2016; 12(8):453-71. doi: 10.1038/nrneph.2016.75 [Crossref] [ Google Scholar]
- Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59(4):1498-509. doi: 10.1046/j.1523-1755.2001.0590041498.x [Crossref] [ Google Scholar]
- Declèves AE, Zolkipli Z, Satriano J, Wang L, Nakayama T, Rogac M. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int 2014; 85(3):611-23. doi: 10.1038/ki.2013.462 [Crossref] [ Google Scholar]
- Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012; 74:351-75. doi: 10.1146/annurev-physiol-020911-153333 [Crossref] [ Google Scholar]
- Qiao YF, Guo WJ, Li L, Shao S, Qiao X, Shao JJ. Melatonin attenuates hypertension-induced renal injury partially through inhibiting oxidative stress in rats. Mol Med Rep 2016; 13(1):21-6. doi: 10.3892/mmr.2015.4495 [Crossref] [ Google Scholar]
- Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc 2017; 76(4):443-54. doi: 10.1017/s0029665117002014 [Crossref] [ Google Scholar]
- Rollins CP, Gallino D, Kong V, Ayranci G, Devenyi GA, Germann J. Contributions of a high-fat diet to Alzheimer’s disease-related decline: a longitudinal behavioural and structural neuroimaging study in mouse models. Neuroimage Clin 2019; 21:101606. doi: 10.1016/j.nicl.2018.11.016 [Crossref] [ Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun 2014; 40:9-17. doi: 10.1016/j.bbi.2014.03.005 [Crossref] [ Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12(12):723-38. doi: 10.1038/nrn3114 [Crossref] [ Google Scholar]
- Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc 2017; 76(4):443-54. doi: 10.1017/s0029665117002014 [Crossref] [ Google Scholar]
- Liu Z, Sanossian N, Starkman S, Avila-Rinek G, Eckstein M, Sharma LK. Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes. Stroke 2021; 52(1):144-51. doi: 10.1161/strokeaha.119.027900 [Crossref] [ Google Scholar]
- Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med 2010; 3:335-43. doi: 10.2147/ijgm.S11926 [Crossref] [ Google Scholar]
- Koenig SM. Pulmonary complications of obesity. Am J Med Sci 2001; 321(4):249-79. doi: 10.1097/00000441-200104000-00006 [Crossref] [ Google Scholar]
- Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med 2018; 12(9):755-67. doi: 10.1080/17476348.2018.1506331 [Crossref] [ Google Scholar]
- Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis 2008; 5(4):233-42. doi: 10.1177/1479972308096978 [Crossref] [ Google Scholar]
- Cardinale F, Ciprandi G, Barberi S, Bernardini R, Caffarelli C, Calvani M. Consensus statement of the Italian society of pediatric allergy and immunology for the pragmatic management of children and adolescents with allergic or immunological diseases during the COVID-19 pandemic. Ital J Pediatr 2020; 46(1):84. doi: 10.1186/s13052-020-00843-2 [Crossref] [ Google Scholar]
- di Palmo E, Filice E, Cavallo A, Caffarelli C, Maltoni G, Miniaci A. Childhood obesity and respiratory diseases: which link?. Children (Basel) 2021; 8(3):177. doi: 10.3390/children8030177 [Crossref] [ Google Scholar]
- Bianco A, Nigro E, Monaco ML, Matera MG, Scudiero O, Mazzarella G. The burden of obesity in asthma and COPD: role of adiponectin. Pulm Pharmacol Ther 2017; 43:20-5. doi: 10.1016/j.pupt.2017.01.004 [Crossref] [ Google Scholar]
- Bosello O, Donataccio MP, Cuzzolaro M. Obesity or obesities? Controversies on the association between body mass index and premature mortality. Eat Weight Disord 2016; 21(2):165-74. doi: 10.1007/s40519-016-0278-4 [Crossref] [ Google Scholar]
- Bianco A, Mazzarella G, Turchiarelli V, Nigro E, Corbi G, Scudiero O. Adiponectin: an attractive marker for metabolic disorders in chronic obstructive pulmonary disease (COPD). Nutrients 2013; 5(10):4115-25. doi: 10.3390/nu5104115 [Crossref] [ Google Scholar]
- Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6(10):772-83. doi: 10.1038/nri1937 [Crossref] [ Google Scholar]
- Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R. Obesity, adipokines and asthma. Allergy 2009; 64(5):770-7. doi: 10.1111/j.1398-9995.2008.01872.x [Crossref] [ Google Scholar]
- McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1 Epidemiology. Thorax 2008; 63(7):649-54. doi: 10.1136/thx.2007.086801 [Crossref] [ Google Scholar]
- McClean KM, Cardwell CR, Kee F. Longitudinal change in BMI and lung function in middle-aged men in Northern Ireland. Ir J Med Sci 2007; 176(Suppl 10):S418. [ Google Scholar]
- Nakajima K, Kubouchi Y, Muneyuki T, Ebata M, Eguchi S, Munakata H. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest 2008; 134(4):712-8. doi: 10.1378/chest.07-3003 [Crossref] [ Google Scholar]
- Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006; 130(3):827-33. doi: 10.1378/chest.130.3.827 [Crossref] [ Google Scholar]
- Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Trevisan M. Pulmonary function and abdominal adiposity in the general population. Chest 2006; 129(4):853-62. doi: 10.1378/chest.129.4.853 [Crossref] [ Google Scholar]
- Wannamethee SG, Shaper AG, Whincup PH. Body fat distribution, body composition, and respiratory function in elderly men. Am J Clin Nutr 2005; 82(5):996-1003. doi: 10.1093/ajcn/82.5.996 [Crossref] [ Google Scholar]
- Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009; 179(6):509-16. doi: 10.1164/rccm.200807-1195OC [Crossref] [ Google Scholar]
- Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 2002; 122(4):1256-63. doi: 10.1378/chest.122.4.1256 [Crossref] [ Google Scholar]
- McDonald VM, Gibson PG, Scott HA, Baines PJ, Hensley MJ, Pretto JJ. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016; 21(5):875-82. doi: 10.1111/resp.12746 [Crossref] [ Google Scholar]
- Cebron Lipovec N, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AM. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. Copd 2016; 13(3):399-406. doi: 10.3109/15412555.2016.1140732 [Crossref] [ Google Scholar]
- Maatman RC, Spruit MA, van Melick PP, Peeters JP, Rutten EP, Vanfleteren LE. Effects of obesity on weight-bearing versus weight-supported exercise testing in patients with COPD. Respirology 2016; 21(3):483-8. doi: 10.1111/resp.12700 [Crossref] [ Google Scholar]
- Bonsaksen T, Fagermoen MS, Lerdal A. Trajectories of physical and mental health among persons with morbid obesity and persons with COPD: a longitudinal comparative study. J Multidiscip Healthc 2016; 9:191-200. doi: 10.2147/jmdh.S102630 [Crossref] [ Google Scholar]
- Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175(7):661-6. doi: 10.1164/rccm.200611-1717OC [Crossref] [ Google Scholar]
- Tay TR, Radhakrishna N, Hore-Lacy F, Smith C, Hoy R, Dabscheck E. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016; 21(8):1384-90. doi: 10.1111/resp.12838 [Crossref] [ Google Scholar]
- Abdul Wahab A, Maarafiya MM, Soliman A, Younes NB, Chandra P. Serum leptin and adiponectin levels in obese and nonobese asthmatic school children in relation to asthma control. J Allergy (Cairo) 2013; 2013:654104. doi: 10.1155/2013/654104 [Crossref] [ Google Scholar]
- Wadsworth S, Sin D, Dorscheid D. Clinical update on the use of biomarkers of airway inflammation in the management of asthma. J Asthma Allergy 2011; 4:77-86. doi: 10.2147/jaa.S15081 [Crossref] [ Google Scholar]
- Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS One 2016; 11(9):e0163677. doi: 10.1371/journal.pone.0163677 [Crossref] [ Google Scholar]
- Cheung YM, Joham A, Marks S, Teede H. The obesity paradox: an endocrine perspective. Intern Med J 2017; 47(7):727-33. doi: 10.1111/imj.13257 [Crossref] [ Google Scholar]
- Faria A, Allen AH, Fox N, Ayas N, Laher I. The public health burden of obstructive sleep apnea. Sleep Sci 2021; 14(3):257-65. doi: 10.5935/1984-0063.20200111 [Crossref] [ Google Scholar]
- Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med 2008; 4(4):333-8. [ Google Scholar]
- Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol (1985) 2007; 102(2):547-56. doi: 10.1152/japplphysiol.00282.2006 [Crossref] [ Google Scholar]
- Sato M, Suzuki M, Suzuki J, Endo Y, Chiba Y, Matsuura M. Overweight patients with severe sleep apnea experience deeper oxygen desaturation at apneic events. J Med Dent Sci 2008; 55(1):43-7. [ Google Scholar]
- Emerenziani S, Guarino MP, Trillo Asensio LM, Altomare A, Ribolsi M, Balestrieri P. Role of overweight and obesity in gastrointestinal disease. Nutrients 2019; 12(1):111. doi: 10.3390/nu12010111 [Crossref] [ Google Scholar]
- Dreyer JL, Liebl AL. Early colonization of the gut microbiome and its relationship with obesity. Hum Microb J 2018; 10:1-5. doi: 10.1016/j.humic.2018.08.002 [Crossref] [ Google Scholar]
- Rogers CJ, Prabhu KS, Vijay-Kumar M. The microbiome and obesity-an established risk for certain types of cancer. Cancer J 2014; 20(3):176-80. doi: 10.1097/ppo.0000000000000049 [Crossref] [ Google Scholar]
- Li Q, Zhang J, Zhou Y, Qiao L. Obesity and gastric cancer. Front Biosci (Landmark Ed) 2012; 17(7):2383-90. doi: 10.2741/4059 [Crossref] [ Google Scholar]
- Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci 2014; 1311:57-76. doi: 10.1111/nyas.12416 [Crossref] [ Google Scholar]
- Goodwin PJ, Chlebowski RT. Obesity and cancer: insights for clinicians. J Clin Oncol 2016; 34(35):4197-202. doi: 10.1200/jco.2016.70.5327 [Crossref] [ Google Scholar]
- Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschöp MH. Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab 2007; 3(10):705-12. doi: 10.1038/ncpendmet0625 [Crossref] [ Google Scholar]
- Steele JR, Riddiford-Harland DL, Mickle KJ. Excessive weight bearing compromises foot structure and function across the lifespan. In: Gefen A, Benayahu D, eds. The Mechanobiology of Obesity and Related Diseases. Cham: Springer; 2015. p. 149-79. doi: 10.1007/8415_2014_175.
- Ursini F, Naty S, Grembiale RD. Fibromyalgia and obesity: the hidden link. Rheumatol Int 2011; 31(11):1403-8. doi: 10.1007/s00296-011-1885-z [Crossref] [ Google Scholar]
- Kadam UT, Jordan K, Croft PR. Clinical comorbidity in patients with osteoarthritis: a case-control study of general practice consulters in England and Wales. Ann Rheum Dis 2004; 63(4):408-14. doi: 10.1136/ard.2003.007526 [Crossref] [ Google Scholar]
- Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) 2008; 32(2):211-22. doi: 10.1038/sj.ijo.0803715 [Crossref] [ Google Scholar]
- Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: a systematic review and meta-analysis of Mendelian randomization studies. BMC Med 2021; 19(1):320. doi: 10.1186/s12916-021-02188-x [Crossref] [ Google Scholar]
- Stallings SP, Kasdan ML, Soergel TM, Corwin HM. A case-control study of obesity as a risk factor for carpal tunnel syndrome in a population of 600 patients presenting for independent medical examination. J Hand Surg Am 1997; 22(2):211-5. doi: 10.1016/s0363-5023(97)80153-0 [Crossref] [ Google Scholar]
- Geha P, de Araujo I, Green B, Small DM. Decreased food pleasure and disrupted satiety signals in chronic low back pain. Pain 2014; 155(4):712-22. doi: 10.1016/j.pain.2013.12.027 [Crossref] [ Google Scholar]
- Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res 2015; 8:399-408. doi: 10.2147/jpr.S55598 [Crossref] [ Google Scholar]
- Wearing SC, Grigg NL, Lau HC, Smeathers JE. Footprint-based estimates of arch structure are confounded by body composition in adults. J Orthop Res 2012; 30(8):1351-4. doi: 10.1002/jor.22058 [Crossref] [ Google Scholar]
- Riddiford-Harland DL, Steele JR, Storlien LH. Does obesity influence foot structure in prepubescent children?. Int J Obes Relat Metab Disord 2000; 24(5):541-4. doi: 10.1038/sj.ijo.0801192 [Crossref] [ Google Scholar]
- Mickle KJ, Steele JR, Munro BJ. The feet of overweight and obese young children: are they flat or fat?. Obesity (Silver Spring) 2006; 14(11):1949-53. doi: 10.1038/oby.2006.227 [Crossref] [ Google Scholar]
- Mauch M, Grau S, Krauss I, Maiwald C, Horstmann T. Foot morphology of normal, underweight and overweight children. Int J Obes (Lond) 2008; 32(7):1068-75. doi: 10.1038/ijo.2008.52 [Crossref] [ Google Scholar]
- Chen JP, Chung MJ, Wang MJ. Flatfoot prevalence and foot dimensions of 5- to 13-year-old children in Taiwan. Foot Ankle Int 2009; 30(4):326-32. doi: 10.3113/fai.2009.0326 [Crossref] [ Google Scholar]
- Yan SH, Zhang K, Tan GQ, Yang J, Liu ZC. Effects of obesity on dynamic plantar pressure distribution in Chinese prepubescent children during walking. Gait Posture 2013; 37(1):37-42. doi: 10.1016/j.gaitpost.2012.05.018 [Crossref] [ Google Scholar]
- Riddiford-Harland DL, Steele JR, Baur LA. Are the feet of obese children fat or flat? Revisiting the debate. Int J Obes (Lond) 2011; 35(1):115-20. doi: 10.1038/ijo.2010.119 [Crossref] [ Google Scholar]
- Monteiro M, Gabriel R, Aranha J, Neves e Castro M, Sousa M, Moreira M. Influence of obesity and sarcopenic obesity on plantar pressure of postmenopausal women. Clin Biomech (Bristol) 2010; 25(5):461-7. doi: 10.1016/j.clinbiomech.2010.01.017 [Crossref] [ Google Scholar]
- Mickle KJ, Steele JR, Munro BJ. Does excess mass affect plantar pressure in young children?. Int J Pediatr Obes 2006; 1(3):183-8. doi: 10.1080/17477160600881734 [Crossref] [ Google Scholar]
- Hills AP, Hennig EM, McDonald M, Bar-Or O. Plantar pressure differences between obese and non-obese adults: a biomechanical analysis. Int J Obes Relat Metab Disord 2001; 25(11):1674-9. doi: 10.1038/sj.ijo.0801785 [Crossref] [ Google Scholar]
- Gravante G, Russo G, Pomara F, Ridola C. Comparison of ground reaction forces between obese and control young adults during quiet standing on a baropodometric platform. Clin Biomech (Bristol) 2003; 18(8):780-2. doi: 10.1016/s0268-0033(03)00123-2 [Crossref] [ Google Scholar]
- Mahaffey R, Morrison SC, Bassett P, Drechsler WI, Cramp MC. Biomechanical characteristics of lower limb gait waveforms: associations with body fat in children. Gait Posture 2018; 61:220-5. doi: 10.1016/j.gaitpost.2018.01.019 [Crossref] [ Google Scholar]
- Dufek JS, Currie RL, Gouws PL, Candela L, Gutierrez AP, Mercer JA. Effects of overweight and obesity on walking characteristics in adolescents. Hum Mov Sci 2012; 31(4):897-906. doi: 10.1016/j.humov.2011.10.003 [Crossref] [ Google Scholar]
- Nantel J, Brochu M, Prince F. Locomotor strategies in obese and non-obese children. Obesity (Silver Spring) 2006; 14(10):1789-94. doi: 10.1038/oby.2006.206 [Crossref] [ Google Scholar]
- Tanamas SK, Wluka AE, Berry P, Menz HB, Strauss BJ, Davies-Tuck M. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res (Hoboken) 2012; 64(2):262-8. doi: 10.1002/acr.20663 [Crossref] [ Google Scholar]
- Stovitz SD, Pardee PE, Vazquez G, Duval S, Schwimmer JB. Musculoskeletal pain in obese children and adolescents. Acta Paediatr 2008; 97(4):489-93. doi: 10.1111/j.1651-2227.2008.00724.x [Crossref] [ Google Scholar]
- Mickle KJ, Steele JR. Obese older adults suffer foot pain and foot-related functional limitation. Gait Posture 2015; 42(4):442-7. doi: 10.1016/j.gaitpost.2015.07.013 [Crossref] [ Google Scholar]
- Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int 2007; 28(9):996-9. doi: 10.3113/fai.2007.0996 [Crossref] [ Google Scholar]
- Irving DB, Cook JL, Young MA, Menz HB. Obesity and pronated foot type may increase the risk of chronic plantar heel pain: a matched case-control study. BMC Musculoskelet Disord 2007; 8:41. doi: 10.1186/1471-2474-8-41 [Crossref] [ Google Scholar]
- Tukker A, Visscher TL, Picavet HS. Overweight and health problems of the lower extremities: osteoarthritis, pain and disability. Public Health Nutr 2009; 12(3):359-68. doi: 10.1017/s1368980008002103 [Crossref] [ Google Scholar]
- Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity (Silver Spring) 2012; 20(7):1491-5. doi: 10.1038/oby.2011.397 [Crossref] [ Google Scholar]
- Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I) Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol 1988; 128(1):179-89. doi: 10.1093/oxfordjournals.aje.a114939 [Crossref] [ Google Scholar]
- Mondelli M, Rossi S, Romano C. Body mass index in meralgia paresthetica: a case-control study. Acta Neurol Scand 2007; 116(2):118-23. doi: 10.1111/j.1600-0404.2007.00814.x [Crossref] [ Google Scholar]
- Hangai M, Kaneoka K, Kuno S, Hinotsu S, Sakane M, Mamizuka N. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J 2008; 8(5):732-40. doi: 10.1016/j.spinee.2007.07.392 [Crossref] [ Google Scholar]
- Steele JR, Coltman CE, McGhee DE. Effects of obesity on breast size, thoracic spine structure and function, upper torso musculoskeletal pain and physical activity in women. J Sport Health Sci 2020; 9(2):140-8. doi: 10.1016/j.jshs.2019.05.003 [Crossref] [ Google Scholar]
- Sachdeva A, Tiwari MK, Shahid M, Varshney A. Unravelling the complex nexus: adiposity, blood pressure, cardiac autonomic function, and arterial stiffness in young adults-an integrated analysis. Pak Heart J 2023; 56(2):215-9. [ Google Scholar]
- Floras JS, Legault L, Morali GA, Hara K, Blendis LM. Increased sympathetic outflow in cirrhosis and ascites: direct evidence from intraneural recordings. Ann Intern Med 1991; 114(5):373-80. doi: 10.7326/0003-4819-114-5-373 [Crossref] [ Google Scholar]
- Miscio G, Guastamacchia G, Brunani A, Priano L, Baudo S, Mauro A. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst 2005; 10(4):354-8. doi: 10.1111/j.1085-9489.2005.00047.x [Crossref] [ Google Scholar]
- Shahwan MJ, Jairoun AA, Farajallah A, Shanabli S. Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab Syndr 2019; 13(4):2387-92. doi: 10.1016/j.dsx.2019.06.009 [Crossref] [ Google Scholar]
- Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res 2015; 8:399-408. doi: 10.2147/jpr.S55598 [Crossref] [ Google Scholar]
- Friedman MA, Brownell KD. Psychological correlates of obesity: moving to the next research generation. Psychol Bull 1995; 117(1):3-20. doi: 10.1037/0033-2909.117.1.3 [Crossref] [ Google Scholar]
- Kalarchian MA, Marcus MD, Levine MD, Courcoulas AP, Pilkonis PA, Ringham RM. Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry 2007; 164(2):328-34. doi: 10.1176/ajp.2007.164.2.328 [Crossref] [ Google Scholar]
- Jones-Corneille LR, Wadden TA, Sarwer DB, Faulconbridge LF, Fabricatore AN, Stack RM. Axis I psychopathology in bariatric surgery candidates with and without binge eating disorder: results of structured clinical interviews. Obes Surg 2012; 22(3):389-97. doi: 10.1007/s11695-010-0322-9 [Crossref] [ Google Scholar]
- Legenbauer T, De Zwaan M, Benecke A, Muhlhans B, Petrak F, Herpertz S. Depression and anxiety: their predictive function for weight loss in obese individuals. Obes Facts 2009; 2(4):227-34. doi: 10.1159/000226278 [Crossref] [ Google Scholar]
- Mitchell JE, Selzer F, Kalarchian MA, Devlin MJ, Strain GW, Elder KA. Psychopathology before surgery in the longitudinal assessment of bariatric surgery-3 (LABS-3) psychosocial study. Surg Obes Relat Dis 2012; 8(5):533-41. doi: 10.1016/j.soard.2012.07.001 [Crossref] [ Google Scholar]
- Rosenberger PH, Henderson KE, Grilo CM. Correlates of body image dissatisfaction in extremely obese female bariatric surgery candidates. Obes Surg 2006; 16(10):1331-6. doi: 10.1381/096089206778663788 [Crossref] [ Google Scholar]
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2003; 158(12):1139-47. doi: 10.1093/aje/kwg275 [Crossref] [ Google Scholar]
- Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health 2000; 90(2):251-7. doi: 10.2105/ajph.90.2.251 [Crossref] [ Google Scholar]
- Goldfield GS, Moore C, Henderson K, Buchholz A, Obeid N, Flament MF. Body dissatisfaction, dietary restraint, depression, and weight status in adolescents. J Sch Health 2010; 80(4):186-92. doi: 10.1111/j.1746-1561.2009.00485.x [Crossref] [ Google Scholar]
- Erermis S, Cetin N, Tamar M, Bukusoglu N, Akdeniz F, Goksen D. Is obesity a risk factor for psychopathology among adolescents?. Pediatr Int 2004; 46(3):296-301. doi: 10.1111/j.1442-200x.2004.01882.x [Crossref] [ Google Scholar]
- Zeller MH, Reiter-Purtill J, Ramey C. Negative peer perceptions of obese children in the classroom environment. Obesity (Silver Spring) 2008; 16(4):755-62. doi: 10.1038/oby.2008.4 [Crossref] [ Google Scholar]
- Keery H, Boutelle K, van den Berg P, Thompson JK. The impact of appearance-related teasing by family members. J Adolesc Health 2005; 37(2):120-7. doi: 10.1016/j.jadohealth.2004.08.015 [Crossref] [ Google Scholar]
- Madowitz J, Knatz S, Maginot T, Crow SJ, Boutelle KN. Teasing, depression and unhealthy weight control behaviour in obese children. Pediatr Obes 2012; 7(6):446-52. doi: 10.1111/j.2047-6310.2012.00078.x [Crossref] [ Google Scholar]
- Whetstone LM, Morrissey SL, Cummings DM. Children at risk: the association between perceived weight status and suicidal thoughts and attempts in middle school youth. J Sch Health 2007; 77(2):59-66. doi: 10.1111/j.1746-1561.2007.00168.x [Crossref] [ Google Scholar]
- Mather AA, Cox BJ, Enns MW, Sareen J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J Psychosom Res 2009; 66(4):277-85. doi: 10.1016/j.jpsychores.2008.09.008 [Crossref] [ Google Scholar]
- Dutton GR, Bodell LP, Smith AR, Joiner TE. Examination of the relationship between obesity and suicidal ideation. Int J Obes (Lond) 2013; 37(9):1282-6. doi: 10.1038/ijo.2012.224 [Crossref] [ Google Scholar]
- Ju YJ, Han KT, Lee TH, Kim W, Park JH, Park EC. Association between weight control failure and suicidal ideation in overweight and obese adults: a cross-sectional study. BMC Public Health 2016; 16:259. doi: 10.1186/s12889-016-2940-1 [Crossref] [ Google Scholar]
- Wagner B, Klinitzke G, Brähler E, Kersting A. Extreme obesity is associated with suicidal behavior and suicide attempts in adults: results of a population-based representative sample. Depress Anxiety 2013; 30(10):975-81. doi: 10.1002/da.22105 [Crossref] [ Google Scholar]
- Wadden TA, Butryn ML, Sarwer DB, Fabricatore AN, Crerand CE, Lipschutz PE. Comparison of psychosocial status in treatment-seeking women with class III vs class I-II obesity. Surg Obes Relat Dis 2006; 2(2):138-45. doi: 10.1016/j.soard.2006.03.016 [Crossref] [ Google Scholar]
- Pawlow LA, O’Neil PM, White MA, Byrne TK. Findings and outcomes of psychological evaluations of gastric bypass applicants. Surg Obes Relat Dis 2005; 1(6):523-7. doi: 10.1016/j.soard.2005.08.007 [Crossref] [ Google Scholar]
- Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009; 17(5):941-64. doi: 10.1038/oby.2008.636 [Crossref] [ Google Scholar]
- Puhl R, Brownell KD. Bias, discrimination, and obesity. Obes Res 2001; 9(12):788-805. doi: 10.1038/oby.2001.108 [Crossref] [ Google Scholar]
- Brownell KD, Puhl RM, Schwartz MB, Rudd LE. Weight Bias: Nature, Consequences, and Remedies. New York, NY: Guilford Publications; 2005.
- Wilson AL, Goldfield GS. Overweight or obese young people are not at increased risk of depression, but young people with depression are at increased risk of obesity. Evid Based Nurs 2014; 17(4):112. doi: 10.1136/eb-2013-101675 [Crossref] [ Google Scholar]
- Sarwer DB, Moore RH, Spitzer JC, Wadden TA, Raper SE, Williams NN. A pilot study investigating the efficacy of postoperative dietary counseling to improve outcomes after bariatric surgery. Surg Obes Relat Dis 2012; 8(5):561-8. doi: 10.1016/j.soard.2012.02.010 [Crossref] [ Google Scholar]
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. APA; 2013.
- Hadjigeorgiou C, Tornaritis M, Savvas S, Solea A, Kafatos A. Obesity and psychological traits associated with eating disorders among Cypriot adolescents: comparison of 2003 and 2010 cohorts. East Mediterr Health J 2012; 18(8):842-9. doi: 10.26719/2012.18.8.842 [Crossref] [ Google Scholar]
- Lundstedt G, Edlund B, Engström I, Thurfjell B, Marcus C. Eating disorder traits in obese children and adolescents. Eat Weight Disord 2006; 11(1):45-50. doi: 10.1007/bf03327743 [Crossref] [ Google Scholar]
- Decaluwé V, Braet C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. Int J Obes Relat Metab Disord 2003; 27(3):404-9. doi: 10.1038/sj.ijo.0802233 [Crossref] [ Google Scholar]
- Decaluwé V, Braet C, Fairburn CG. Binge eating in obese children and adolescents. Int J Eat Disord 2003; 33(1):78-84. doi: 10.1002/eat.10110 [Crossref] [ Google Scholar]
- Cena H, Stanford FC, Ochner L, Fonte ML, Biino G, De Giuseppe R. Association of a history of childhood-onset obesity and dieting with eating disorders. Eat Disord 2017; 25(3):216-29. doi: 10.1080/10640266.2017.1279905 [Crossref] [ Google Scholar]
- Kalarchian MA, Wilson GT, Brolin RE, Bradley L. Effects of bariatric surgery on binge eating and related psychopathology. Eat Weight Disord 1999; 4(1):1-5. doi: 10.1007/bf03376581 [Crossref] [ Google Scholar]
- Kalarchian MA, Wilson GT, Brolin RE, Bradley L. Binge eating in bariatric surgery patients. Int J Eat Disord 1998; 23(1):89-92. doi: 10.1002/(sici)1098-108x(199801)23:1<89::aideat11>3.0.co;2-i [Crossref] [ Google Scholar]
- Adami GF, Gandolfo P, Bauer B, Scopinaro N. Binge eating in massively obese patients undergoing bariatric surgery. Int J Eat Disord 1995; 17(1):45-50. doi: 10.1002/1098-108x(199501)17:1<45::aid-eat2260170106>3.0.co;2-s [Crossref] [ Google Scholar]
- Hsu LK, Sullivan SP, Benotti PN. Eating disturbances and outcome of gastric bypass surgery: a pilot study. Int J Eat Disord 1997; 21(4):385-90. doi: 10.1002/(sici)1098-108x(1997)21:4<385::aid-eat12>3.0.co;2-y [Crossref] [ Google Scholar]
- Mitchell JE, King WC, Pories W, Wolfe B, Flum DR, Spaniolas K. Binge eating disorder and medical comorbidities in bariatric surgery candidates. Int J Eat Disord 2015; 48(5):471-6. doi: 10.1002/eat.22389 [Crossref] [ Google Scholar]
- Allison KC, Wadden TA, Sarwer DB, Fabricatore AN, Crerand CE, Gibbons LM. Night eating syndrome and binge eating disorder among persons seeking bariatric surgery: prevalence and related features. Obesity (Silver Spring) 2006; 14 Suppl 2:77S-82S. doi: 10.1038/oby.2006.286 [Crossref] [ Google Scholar]
- Stunkard AJ, Allison KC. Two forms of disordered eating in obesity: binge eating and night eating. Int J Obes Relat Metab Disord 2003; 27(1):1-12. doi: 10.1038/sj.ijo.0802186 [Crossref] [ Google Scholar]
- Kambey PA, Kodzo LD, Serojane F, Oluwasola BJ. The bi-directional association between bipolar disorder and obesity: evidence from meta and bioinformatics analysis. Int J Obes (Lond) 2023; 47(6):443-52. doi: 10.1038/s41366-023-01277-6 [Crossref] [ Google Scholar]
- Pickering RP, Grant BF, Chou SP, Compton WM. Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 2007; 68(7):998-1009. doi: 10.4088/jcp.v68n0704 [Crossref] [ Google Scholar]
- Dudek D, Siwek M, Jaeschke R, Dembińska-Kieć A, Arciszewska A, Hebal F. Relationships between obesity, bipolar spectrum features, and personality traits: a case-control study. Eur Rev Med Pharmacol Sci 2015; 19(22):4235-40. [ Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63(7):824-30. doi: 10.1001/archpsyc.63.7.824 [Crossref] [ Google Scholar]
- Chouinard VA, Pingali SM, Chouinard G, Henderson DC, Mallya SG, Cypess AM. Factors associated with overweight and obesity in schizophrenia, schizoaffective and bipolar disorders. Psychiatry Res 2016; 237:304-10. doi: 10.1016/j.psychres.2016.01.024 [Crossref] [ Google Scholar]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253.
- Guidelines (2013) for managing overweight and obesity in adults. Preface to the Expert Panel Report (comprehensive version which includes systematic evidence review, evidence statements, and recommendations). Obesity (Silver Spring). 2014;22 Suppl 2:S40. doi: 10.1002/oby.20822.
- Lee EY, Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med 2018; 12(6):658-66. doi: 10.1007/s11684-018-0640-1 [Crossref] [ Google Scholar]
- Sandouk Z, Lansang MC. Diabetes with obesity--is there an ideal diet?. Cleve Clin J Med 2017; 84(7 Suppl 1):S4-14. doi: 10.3949/ccjm.84.s1.02 [Crossref] [ Google Scholar]
- Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci 2013; 1281(1):141-59. doi: 10.1111/j.1749-6632.2012.06785.x [Crossref] [ Google Scholar]
- Carlson SA, Fulton JE, Schoenborn CA, Loustalot F. Trend and prevalence estimates based on the 2008 Physical Activity Guidelines for Americans. Am J Prev Med 2010; 39(4):305-13. doi: 10.1016/j.amepre.2010.06.006 [Crossref] [ Google Scholar]
- Garland T Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 2011; 214(Pt 2):206-29. doi: 10.1242/jeb.048397 [Crossref] [ Google Scholar]
- Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012; 13(8):659-80. doi: 10.1111/j.1467-789X.2011.00982.x [Crossref] [ Google Scholar]
- Nguyen B, Clements J. Obesity management among patients with type 2 diabetes and prediabetes: a focus on lifestyle modifications and evidence of antiobesity medications. Expert Rev Endocrinol Metab 2017; 12(5):303-13. doi: 10.1080/17446651.2017.1367285 [Crossref] [ Google Scholar]
- Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S. Interventions for preventing obesity in children. Cochrane Database Syst Rev 2019; 7(7):CD001871. doi: 10.1002/14651858.CD001871.pub4 [Crossref] [ Google Scholar]
- Wolfenden L, Jones J, Williams CM, Finch M, Wyse RJ, Kingsland M. Strategies to improve the implementation of healthy eating, physical activity and obesity prevention policies, practices or programmes within childcare services. Cochrane Database Syst Rev 2016; 10(10):CD011779. doi: 10.1002/14651858.CD011779.pub2 [Crossref] [ Google Scholar]
- Maheshwari A, Maheshwari G. Aging population in Vietnam: challenges, implications, and policy recommendations. Int J Aging 2024; 2(1):e1. doi: 10.34172/ija.2024.e1 [Crossref] [ Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011; 364(25):2392-404. doi: 10.1056/NEJMoa1014296 [Crossref] [ Google Scholar]
- Jacobson MF, Brownell KD. Small taxes on soft drinks and snack foods to promote health. Am J Public Health 2000; 90(6):854-7. doi: 10.2105/ajph.90.6.854 [Crossref] [ Google Scholar]
- Struben J, Chan D, Dubé L. Policy insights from the nutritional food market transformation model: the case of obesity prevention. Ann N Y Acad Sci 2014; 1331:57-75. doi: 10.1111/nyas.12381 [Crossref] [ Google Scholar]
- Swinburn B, Egger G. Preventive strategies against weight gain and obesity. Obes Rev 2002; 3(4):289-301. doi: 10.1046/j.1467-789x.2002.00082.x [Crossref] [ Google Scholar]
- Lal A, Mantilla-Herrera AM, Veerman L, Backholer K, Sacks G, Moodie M. Correction: modelled health benefits of a sugar-sweetened beverage tax across different socioeconomic groups in Australia: a cost-effectiveness and equity analysis. PLoS Med 2020; 17(7):e1003310. doi: 10.1371/journal.pmed.1003310 [Crossref] [ Google Scholar]
- Abdi Beshir S, Ahmed Elnour A, Soorya A, Parveen Mohamed A, Sir Loon Goh S, Hussain N. A narrative review of approved and emerging anti-obesity medications. Saudi Pharm J 2023; 31(10):101757. doi: 10.1016/j.jsps.2023.101757 [Crossref] [ Google Scholar]
- Tchang BG, Aras M, Kumar RB, Aronne AJ. Pharmacologic treatment of overweight and obesity in adults. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2000. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279038/. Updated August 2, 2021.
- Haqq AM, Chung WK, Dollfus H, Haws RM, Martos-Moreno G, Poitou C. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol 2022; 10(12):859-68. doi: 10.1016/s2213-8587(22)00277-7 [Crossref] [ Google Scholar]
- Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, Mantzoros CS. Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine 2023; 58:101882. doi: 10.1016/j.eclinm.2023.101882 [Crossref] [ Google Scholar]
- Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord 1997; 21 Suppl 3:S12-23. [ Google Scholar]
- Bansal AB, Al Khalili Y. Orlistat. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542202/. Updated December 11, 2022.
- Ballinger A. Orlistat in the treatment of obesity. Expert Opin Pharmacother 2000; 1(4):841-7. doi: 10.1517/14656566.1.4.841 [Crossref] [ Google Scholar]
- Johnson DB, Quick J. Topiramate and phentermine. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482165/. Updated March 27, 2023.
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013; 21(11):2163-71. doi: 10.1002/oby.20584 [Crossref] [ Google Scholar]
- Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother 2013; 47(3):340-9. doi: 10.1345/aph.1R501 [Crossref] [ Google Scholar]
- Lei XG, Ruan JQ, Lai C, Sun Z, Yang X. Efficacy and safety of phentermine/topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity (Silver Spring) 2021; 29(6):985-94. doi: 10.1002/oby.23152 [Crossref] [ Google Scholar]
- Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA 2015; 313(12):1213-4. doi: 10.1001/jama.2015.1617 [Crossref] [ Google Scholar]
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013; 21(5):935-43. doi: 10.1002/oby.20309 [Crossref] [ Google Scholar]
- Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011; 19(1):110-20. doi: 10.1038/oby.2010.147 [Crossref] [ Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010; 376(9741):595-605. doi: 10.1016/s0140-6736(10)60888-4 [Crossref] [ Google Scholar]
- Hasanzad M, Sarhangi N, Nikfar S, Ostad SN, Aghaei Meybodi HR. A narrative review of current trends in liraglutide: insights into the unmet needs in management of type 2 diabetes and obesity. J Diabetes Metab Disord 2020; 19(2):1863-72. doi: 10.1007/s40200-020-00619-9 [Crossref] [ Google Scholar]
- Alruwaili H, Dehestani B, Le Roux CW. Clinical impact of liraglutide as a treatment of obesity. Clin Pharmacol 2021; 13:53-60. doi: 10.2147/cpaa.S276085 [Crossref] [ Google Scholar]
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M. A randomized, controlled trial of 30 mg of liraglutide in weight management. N Engl J Med 2015; 373(1):11-22. doi: 10.1056/NEJMoa1411892 [Crossref] [ Google Scholar]
- Lin Q, Xue Y, Zou H, Ruan Z, Ung COL, Hu H. Efficacy and safety of liraglutide for obesity and people who are overweight: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol 2022; 15(12):1461-9. doi: 10.1080/17512433.2022.2130760 [Crossref] [ Google Scholar]
- Jeong D, Priefer R. Anti-obesity weight loss medications: Short-term and long-term use. Life Sci 2022; 306:120825. doi: 10.1016/j.lfs.2022.120825 [Crossref] [ Google Scholar]
- Markham A. Setmelanotide: First Approval. Drugs 2021; 81(3):397-403. doi: 10.1007/s40265-021-01470-9 [Crossref] [ Google Scholar]
- Hussain A, Farzam K. Setmelanotide. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK589641/. Updated 2023 July 2, 2023.
- Ryan DH. Next generation antiobesity medications: setmelanotide, semaglutide, tirzepatide and bimagrumab: what do they mean for clinical practice?. J Obes Metab Syndr 2021; 30(3):196-208. doi: 10.7570/jomes21033 [Crossref] [ Google Scholar]
- Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol 2020; 8(12):960-70. doi: 10.1016/s2213-8587(20)30364-8 [Crossref] [ Google Scholar]
- Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol Metab 2017; 6(10):1321-9. doi: 10.1016/j.molmet.2017.06.015 [Crossref] [ Google Scholar]
- Anam M, Maharjan S, Amjad Z, Abaza A, Vasavada AM, Sadhu A. Efficacy of semaglutide in treating obesity: a systematic review of randomized controlled trials (RCTs). Cureus 2022; 14(12):e32610. doi: 10.7759/cureus.32610 [Crossref] [ Google Scholar]
- Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, Rosenstock J. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023; 402(10403):705-19. doi: 10.1016/s0140-6736(23)01185-6 [Crossref] [ Google Scholar]
- Ghusn W, De la Rosa A, Sacoto D, Cifuentes L, Campos A, Feris F. Weight loss outcomes associated with semaglutide treatment for patients with overweight or obesity. JAMA Netw Open 2022; 5(9):e2231982. doi: 10.1001/jamanetworkopen.2022.31982 [Crossref] [ Google Scholar]
- Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med 2022; 28(10):2083-91. doi: 10.1038/s41591-022-02026-4 [Crossref] [ Google Scholar]
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020; 5(17):e140532. doi: 10.1172/jci.insight.140532 [Crossref] [ Google Scholar]
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B. Tirzepatide once weekly for the treatment of obesity. N Engl J Med 2022; 387(3):205-16. doi: 10.1056/NEJMoa2206038 [Crossref] [ Google Scholar]
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385(6):503-15. doi: 10.1056/NEJMoa2107519 [Crossref] [ Google Scholar]
- Greenway FL, Aronne LJ, Raben A, Astrup A, Apovian CM, Hill JO. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity (Silver Spring) 2019; 27(2):205-16. doi: 10.1002/oby.22347 [Crossref] [ Google Scholar]
- US Food and Drug Administration. Is the Product a Medical Device? Available from: https://www.fda.gov/Medicaldevices/Deviceregulationandguidance/Overview/Classifyyourdevice/Ucm051512.htm. Updated March 22, 2018. Accessed July 17, 2018.
- Gambioli R, Lepore E, Biondo FG, Bertolani L, Unfer V. Risks and limits of bariatric surgery: old solutions and a new potential option. Eur Rev Med Pharmacol Sci 2023; 27(12):5831-40. doi: 10.26355/eurrev_202306_32822 [Crossref] [ Google Scholar]
- English WJ, DeMaria EJ, Hutter MM, Kothari SN, Mattar SG, Brethauer SA. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis 2020; 16(4):457-63. doi: 10.1016/j.soard.2019.12.022 [Crossref] [ Google Scholar]
- Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA 2020; 324(9):879-87. doi: 10.1001/jama.2020.12567 [Crossref] [ Google Scholar]
- NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115(12):956-61.
- Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis 2016; 12(6):1144-62. doi: 10.1016/j.soard.2016.05.018 [Crossref] [ Google Scholar]
- Aminian A, Brethauer SA, Andalib A, Nowacki AS, Jimenez A, Corcelles R. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg 2017; 266(4):650-7. doi: 10.1097/sla.0000000000002407 [Crossref] [ Google Scholar]
- Shen SC, Wang W, Tam KW, Chen HA, Lin YK, Wang SY. Validating risk prediction models of diabetes remission after sleeve gastrectomy. Obes Surg 2019; 29(1):221-9. doi: 10.1007/s11695-018-3510-7 [Crossref] [ Google Scholar]
- Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA 2018; 319(3):255-65. doi: 10.1001/jama.2017.20897 [Crossref] [ Google Scholar]
- Kang JH, Le QA. Effectiveness of bariatric surgical procedures: a systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017; 96(46):e8632. doi: 10.1097/md.0000000000008632 [Crossref] [ Google Scholar]
- Uhe I, Douissard J, Podetta M, Chevallay M, Toso C, Jung MK. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity (Silver Spring) 2022; 30(3):614-27. doi: 10.1002/oby.23338 [Crossref] [ Google Scholar]
- Gu L, Huang X, Li S, Mao D, Shen Z, Khadaroo PA. A meta-analysis of the medium- and long-term effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. BMC Surg 2020; 20(1):30. doi: 10.1186/s12893-020-00695-x [Crossref] [ Google Scholar]
- Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MA. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015; 150(10):931-40. doi: 10.1001/jamasurg.2015.1534 [Crossref] [ Google Scholar]
- Cummings DE, Arterburn DE, Westbrook EO, Kuzma JN, Stewart SD, Chan CP. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016; 59(5):945-53. doi: 10.1007/s00125-016-3903-x [Crossref] [ Google Scholar]
- Ding SA, Simonson DC, Wewalka M, Halperin F, Foster K, Goebel-Fabbri A. Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015; 100(7):2546-56. doi: 10.1210/jc.2015-1443 [Crossref] [ Google Scholar]
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299(3):316-23. doi: 10.1001/jama.299.3.316 [Crossref] [ Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017; 376(7):641-51. doi: 10.1056/NEJMoa1600869 [Crossref] [ Google Scholar]
- Ding L, Fan Y, Li H, Zhang Y, Qi D, Tang S. Comparative effectiveness of bariatric surgeries in patients with obesity and type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. Obes Rev 2020; 21(8):e13030. doi: 10.1111/obr.13030 [Crossref] [ Google Scholar]
- Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese type 2 diabetes. Surg Obes Relat Dis 2011; 7(4):433-47. doi: 10.1016/j.soard.2011.05.013 [Crossref] [ Google Scholar]
-
Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. JAMA 2019;321(17):1720-1. doi: 10.1001/jama.2019.2702.
- Ikramuddin S, Billington CJ, Lee WJ, Bantle JP, Thomas AJ, Connett JE. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol 2015; 3(6):413-22. doi: 10.1016/s2213-8587(15)00089-3 [Crossref] [ Google Scholar]
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015; 386(9997):964-73. doi: 10.1016/s0140-6736(15)00075-6 [Crossref] [ Google Scholar]
- Liu DF, Ma ZY, Zhang CS, Lin Q, Li MW, Su KZ. The effects of bariatric surgery on dyslipidemia and insulin resistance in overweight patients with or without type 2 diabetes: a systematic review and network meta-analysis. Surg Obes Relat Dis 2021; 17(9):1655-72. doi: 10.1016/j.soard.2021.04.005 [Crossref] [ Google Scholar]
- Heneghan HM, Meron-Eldar S, Brethauer SA, Schauer PR, Young JB. Effect of bariatric surgery on cardiovascular risk profile. Am J Cardiol 2011; 108(10):1499-507. doi: 10.1016/j.amjcard.2011.06.076 [Crossref] [ Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292(14):1724-37. doi: 10.1001/jama.292.14.1724 [Crossref] [ Google Scholar]
- Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351(26):2683-93. doi: 10.1056/NEJMoa035622 [Crossref] [ Google Scholar]
- Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377(1):13-27. doi: 10.1056/NEJMoa1614362 [Crossref] [ Google Scholar]
- Hua SV, Collis CE, Block JP. Developing effective strategies for obesity prevention. Gastroenterol Clin North Am 2023; 52(2):469-82. doi: 10.1016/j.gtc.2023.03.013 [Crossref] [ Google Scholar]
- Long MW, Tobias DK, Cradock AL, Batchelder H, Gortmaker SL. Systematic review and meta-analysis of the impact of restaurant menu calorie labeling. Am J Public Health 2015; 105(5):e11-24. doi: 10.2105/ajph.2015.302570 [Crossref] [ Google Scholar]
- Bleich SN, Economos CD, Spiker ML, Vercammen KA, VanEpps EM, Block JP. A systematic review of calorie labeling and modified calorie labeling interventions: impact on consumer and restaurant behavior. Obesity (Silver Spring) 2017; 25(12):2018-44. doi: 10.1002/oby.21940 [Crossref] [ Google Scholar]
- Giesen JC, Payne CR, Havermans RC, Jansen A. Exploring how calorie information and taxes on high-calorie foods influence lunch decisions. Am J Clin Nutr 2011; 93(4):689-94. doi: 10.3945/ajcn.110.008193 [Crossref] [ Google Scholar]
- Franck C, Grandi SM, Eisenberg MJ. Taxing junk food to counter obesity. Am J Public Health 2013; 103(11):1949-53. doi: 10.2105/ajph.2013.301279 [Crossref] [ Google Scholar]
- Itria A, Borges SS, Rinaldi AE, Nucci LB, Enes CC. Taxing sugar-sweetened beverages as a policy to reduce overweight and obesity in countries of different income classifications: a systematic review. Public Health Nutr 2021; 24(16):5550-60. doi: 10.1017/s1368980021002901 [Crossref] [ Google Scholar]
- Wanjau MN, Kivuti-Bitok LW, Aminde LN, Veerman JL. The health and economic impact and cost effectiveness of interventions for the prevention and control of overweight and obesity in Kenya: a stakeholder engaged modelling study. Cost Eff Resour Alloc 2023; 21(1):69. doi: 10.1186/s12962-023-00467-3 [Crossref] [ Google Scholar]
- Pate RR, Flynn JI, Dowda M. Policies for promotion of physical activity and prevention of obesity in adolescence. J Exerc Sci Fit 2016; 14(2):47-53. doi: 10.1016/j.jesf.2016.07.003 [Crossref] [ Google Scholar]